SECTION -A
Multiple Choice Questions :
1) Write the answer of following multiple choice questions in the given answer book
i) SI unit of conductivity is –
(A) S
(B) Ω
(C) Scm-1
(D) Sm-1
Ans (D) Sm-1
ii) Element showing the highest number of oxidation states is –
(A) Mn
(B) Ni
C) Fe
(D) Cr
Ans (A) Mn
iii) Oxidation state of Fe in [Fe(Co)5] is-
(A) 0
(B) +2
(C) +3
(D) +4
Ans (B) +2
iv) The chemical formula of chromite ore is –
(A) MnO2
(B) Na2Cr204
(C) FeCr204
(D) Na2CrO4
Ans (C) FeCr204
v) Number of moles of precipitated AgCl on adding excess silver nitrate solution in the solution of one mole of CoCl3.5NH3 is –
(A) 1
(B) 2
(C) 3
(D) 5
Ans (C) 3
vi) Metal present in haemoglobin is –
(A) Mn
(B) Fe
(C) Co
(D) Ni
Ans (B) Fe
vi) Ambidentate ligand is –
(A) Cl–
(B) H2O
(C) NH3
(D) NO2–
Ans (D) NO2–
vii) The compound obtained from meadow sweet is –
(A) Salicyl aldehyde
(B) Cinnamaldehyde
(C) Valeraldehyde
(D) Vanillin
Ans (A) Salicyl aldehyde
ix) IUPAC name of 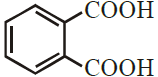 is –
is –
(A) Phthalic acid
(B) Benzene -1, 2- dicarboxylic acid
(C) Benzene -1, 2-dioic acid
(D) Phenyl -1, 2- dicarboxylic acid
Ans (C) Benzene -1, 2-dioic acid
x) Change occurs in hybridisation state of carbonyl carbon in nucleophilic addition reaction is –
(A) sp2 to sp
(B) sp to sp2
(C) sp2 to sp3
(D) sp3 to sp2
Ans (A) sp2 to sp
xi) The hybridisation state of nitrogen intrimethylamine is-
(A) sp
(B) sp2
(C) sp3
(D) dsp2
Ans (C) sp3
xii) The compound showing highest basic strength in aqueous solution is –
(A) (C2H5)3N
(B) (C2H5)2NH
(C) C2H5NH2
(D) NH3
Ans (D) NH3
xiii) Secondary amine among the following is –
(A) Propan -2- amine
(B) Pentan -3-amine
(C) N-Methylethanamine
(D) N,N- Dimethylmethanamine
Ans (D) N,N- Dimethylmethanamine
xiv) Globular protein is –
(A) Insulin
(B) Keratin
(C) Myosin
(D) Collagen
Ans (A) Insulin
xv) Glucose is –
(A) An aldopentose
(B) A ketopentose
(C) An aldohexose
(D) A ketohexose
Ans (C) An aldohexose
xvi) Milk sugar is –
(A) Sucrose
(B) Lactose
(C) Maltose
(D) Galactose
Ans (B) Lactose
2) Fill in the blanks
i) The unit of molarity is …………………..
Ans moles per liter, ( mol/L)
ii)The mathematical form of Henry’s Law is………………………..
Ans C= KH.P
iii) The kinetic energy of maximum fraction of reactant molecules is called …………….
Ans Activation Energy
iv) …………………and actinoids are called as inner transition elements.
Ans Lanthanoids
v) …………………is coordination sphere in K4[Fe(CN)6].
Ans [Fe(CN)6]⁴⁻
vi) In benzylic alcohols -OH group is bonded with …………….. hybridised carbon.
Ans sp2
vii) Glucose is converted to ethanol in presence of ……………………. enzyme.
Ans Zymase
viii) Sodium benzoate is used as ………………….
Ans Food Preservative
ix) Deficiency of vitamin…………………….. causes Beri-Beri disease.
Ans Vitamin B
x) The name of Sugar present in RNA is ……………….
Ans Ribose
3) Very short answer questions:
i) Define saturated solution.
Ans A saturated solution is a solution in which the maximum amount of solute has been dissolved at a given temperature, resulting in equilibrium between the dissolved and undissolved solute.
ii) Write names of solute and solvent present in sodium amalgam solution.
Ans Solute: Sodium (Na)
Solvent: Mercury (Hg)
iii) Rate K[A]½[B]½ Calculate the overall order of a reaction which has above rate expression.
Ans ½ + ½ = 1
So over all order – First order
iv) Write directive influence of -OCH3 group present in anisole for electrophilic substitution reaction.
Ans: It directs incoming electrophiles to attack at ortho and para positions with respect to the methoxy (-OCH3) group.
v) Write chemical name of white precipitate obtained on the reaction of phenol with bromine water.
Ans 2,4,6-tribromophenol.
vi) Write chemical name of NH2-NHCONH2
Ans Urea
vii) Write name of the product obtained from the reaction between benzenediazonium chloride and phenol.
Ans Coupling Reactions : Benzene diazonium chloride reacts with phenol in which the phenol molecule at its para position is coupled with the diazonium salt to form p-hydroxyazobenzene.

viii) Write isocyanide test for primary amines.
Ans Carbylamine reaction : Aliphatic and aromatic primary amines on heating with chloroform and ethanolic potassium hydroxide form isocyanides or carbylamines which are foul smelling substances. This reaction is known as carbylamines reaction or isocyanide test.
SECTION -B
Short answer type questions :
4) Calculate the mole fraction of gas A in the solution made on mixing 0.5 moles of gas A and 4.5 moles of gas B.
Ans To calculate the mole fraction of gas A in the solution, we need to first find the total number of moles in the solution, and then divide the moles of gas A by the total moles.
Given:
Moles of gas A = 0.5
Moles of gas B = 4.5
Total moles in the solution = Moles of gas A + Moles of gas B = 0.5 + 4.5 = 5 moles
Mole fraction of gas A = (Moles of gas A) / (Total moles)
= 0.5 / 5
= 0.1
So, the mole fraction of gas A in the solution is 0.1.
5) 0.05 moles of ethanoic acid is dissolved in 250g benzene. Calculate the molality of the solution.
Ans To calculate the molality of the solution, we use the formula:
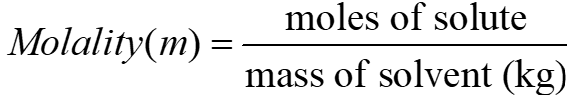
Given:
Moles of ethanoic acid CH3COOH = 0.05 moles-
Mass of benzene C6H6 = 250 g = 0.25 kg
Using the formula:
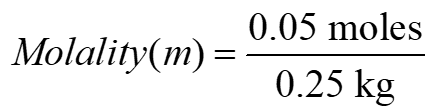
= 0.2 mol/kg
So, the molality of the solution is 0.2mol/kg.
6) Draw the Daniell cell.
Ans 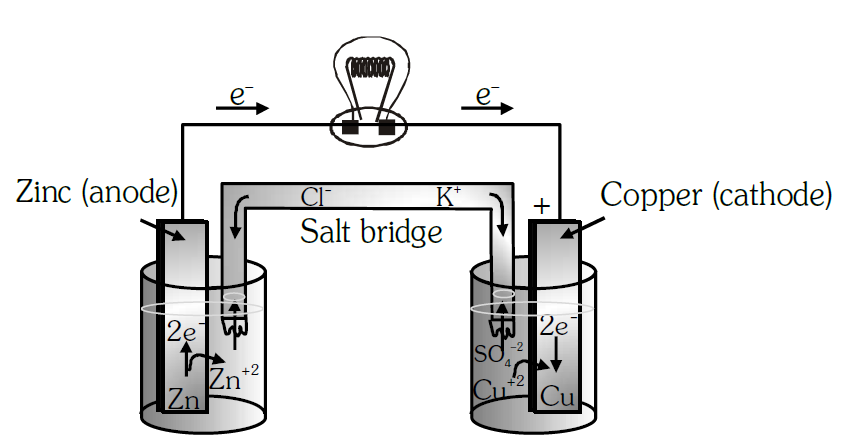
7) Rate constant of a first order reaction is 1.386×10-14 S-1 calculate the half-life of the reaction.
Ans Half life of a first order reaction = ![]()
= ![]() =
= ![]() =
= ![]() S
S
8) Write any three physical characteristics of interstitial compounds.
Ans (i) They have high melting points in comparison to pure metals.
(ii) They are very hard.
(iii) They retain metallic conductivity.
9) Why transition metals exhibit catalytic properties? Explain.
Ans The catalytic activity of the transition elements can be explained by two basic facts.
(a) Owing to their ability to show variable oxidation states and form complexes,
transition metals form unstable intermediate compounds. Thus, they provide a new
path with lower activation energy, Ea, for the reaction.
(b) Transition metals also provide a suitable surface for the reactions to occur.
10) Despite being an electron withdrawing group why Cl-group is ortho-and para directing in electrophilic aromatic substitution? Give reason.
Ans The -I effect is stronger than the +R effect. This means overall, chlorine still deactivates the ring. However, the +R effect is enough to overcome the deactivating effect at the ortho and para positions.
11)  [A]
[A]
Ans 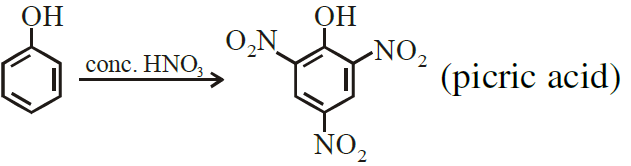
Write IUPAC name and chemical formula of [A] in the above reaction.
IUPAC Name of picric acid 2,4,6-trinitrophenol
12) Compound [x] is formed on heating ethanol with conc. H2SO4 at 413k temperature. Write IUPAC name of [x] and chemical equation of the reaction involved.
Ans ![]()
The IUPAC name of ethene is ethylene.
13) 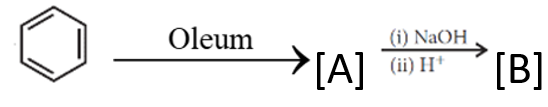
Write chemical formula of [A] and [B] in above reaction sequence.
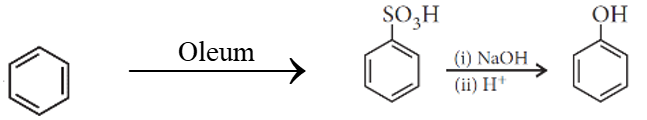
[A] 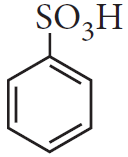 [B]
[B] 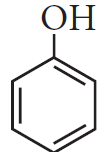
14) Compare basic strength of aniline with ammonia.
Ans In gaseous phase, the order of basicity of amines is 3° amine > 2° amine > 1° amine > NH3.
15) Draw the double strand helix structure DNA.
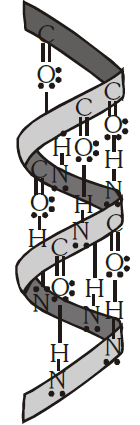
SECTION -C
Long answer type questions :
16 i) Define order of reaction.
Ans Order of reaction : The sum of powers of the molar concentration of the reactants in the rate law expression is called the order of that chemical reaction. Order of reaction can be 0,1,2,3… and even a fraction.
Rate = k[A]x [B]y ⇒ Over all order of reaction n = x + y
ii) Explain pseudo first order reaction by giving appropriate example.
Ans Pseudo first order reaction : Such reaction whose order of reaction is one but molecularity are different then it is called as pseudo first order reaction.
eg. Inversion of cane sugar :
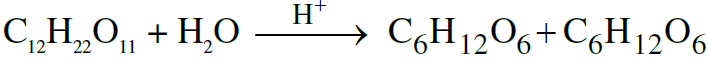
Rate ∝ [C12H22O11]
order of reaction = 1 ⇒ Molecularity = 1 + 1 = 2
OR
i) Define molecularity of a reaction.
Ans Molecularity : The total number of molecules, atoms, ion taking part in the chemical reaction is known as molecularity of reaction. If one, two, three molecules take part in the reaction then the reaction are known as unimolecular, bimolecular, trimolecular respectively.
eg. H2O2 → H2O + O2 unimolecular
H2 + I2 → 2HI bimolecular
ii) Explain the role of activation energy in the feasibility of a chemical reaction.
Ans Lower the activation energy faster is the reaction.
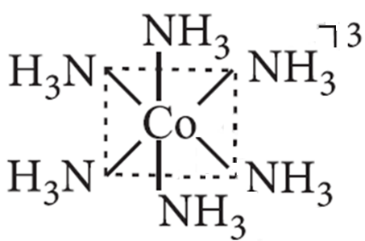
17. Explain the geometry and the magnetic nature of complex [Co(NH3)6]3+ on the basis of valence bond theory.
Ans ![]()
Hybridisation – d2sp3
Structure – Octahedral (low spin)
Nature – Diamagnetic
OR
i) Write IUPAC name of an acid present in vinegar.
Ans Ethanoic acid.
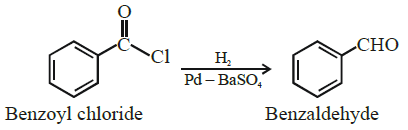
ii) Explain the Rosenmund reduction by giving suitable chemical equation.
Rosenmund Reduction : Acyl chloride (acid chloride) is hydrogenated over catalyst, palladium on barium sulphate. This reaction is called Rosenmund reduction.
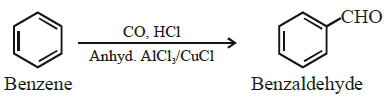
iii) 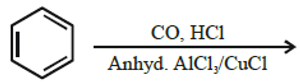 [Z]
[Z]
Write chemical formula of [Z] in above reaction.
Ans Benzaldehyde
SECTON – D
Essay type questions:
18) Explain-
i) The boiling points of isomeric heloalkanes decrease with increase in branching
ii) The melting point of p – isomer is higher than those of o – and m – isomers of isomeric dichlorobenzenes.
iii) The racemic mixture of a optically active compound is always optically inactive.
OR
Convert the following in single step (Write chemical equation only)
i) Benzenediazoniumchloride to iodobenzene.
ii) Chlorobenzene to diphenyl.
iii) Chloroethane to ethene
19) i) Describe the construction of standard hydrogen electrode.
ii) Write equations of cell reactions taking place at anode and cathode during use of dry cell.
OR
i) How does corrosion of iron occurs in presence of water and air? Describe.
ii) Write equations of cell reactions taking place at anode and cathode during use of mercury cell.
20) i)Write IUPAC name of an acid present in red-ant sting.
ii) Explain the Cannizzaro reaction by giving suitable chemical equation.
iii) CH3COONa ![]() [A] + Na2CO3. Write chemical formula of [A] in above reaction.
[A] + Na2CO3. Write chemical formula of [A] in above reaction.
OR
Explain the geometry and the magnetic nature of complex [CoF6]3- on the basis of valence bond theory.
