Chemical Kinetics
Chemical kinetics : It is the branch of chemistry which deals with the study of reaction rates and their mechanisms.
Rate of a reaction : The rate of a reaction can be defined as the change in concentration of a reactant or a product in unit time. For the reaction,
R → P
Rate = ![]() or
or ![]()
Units of rate : Concentration time–1 i.e., mol L–1 s–1 or atm s–1 for gaseous reactions.
Average rate of reaction : It is the average value during a large time interval.
![]()
Rate law and rate constant : The equation that correlates the rate of reaction with concentration of reactants is known as rate law.
|
Molecularity of reaction |
Order of reaction |
|
It is the total number of species taking part in a chemical reaction. |
It is the sum of the powers of the concentration terms of reacting species in the rate law equation. |
|
It is a theoretical concept. |
It is an experimental quantity. |
|
It is derived from the mechanism of reaction. |
It is derived from the rate expression. |
|
It can neither be zero nor fractional. It is always a whole number. |
It may be zero, fractional or an integer (may range from 0 to 3). |
|
It is applicable only to elementary reactions. The overall molecularity of a complex reaction has no significance. |
It is applicable to elementary as well as complex reactions. |
Half life of reaction : The time in which the
concentration of a reactant is reduced to one
half of its initial concentration is called half
life of the reaction.
![]()
where n is the order of the reaction.
Pseudo first order reactions : Those reactions which are not truly of the first order but under certain conditions become reactions of the first order are called pseudo first order reactions. e.g.
Acid hydrolysis of ethyl acetate :
CH3COOC2H5 + H2O ![]() CH3COOH + C2H5OH
CH3COOH + C2H5OH
Rate = k′[CH3COOC2H5][H2O]
= k[CH3COOC2H5]
where, k = k′[H2O]
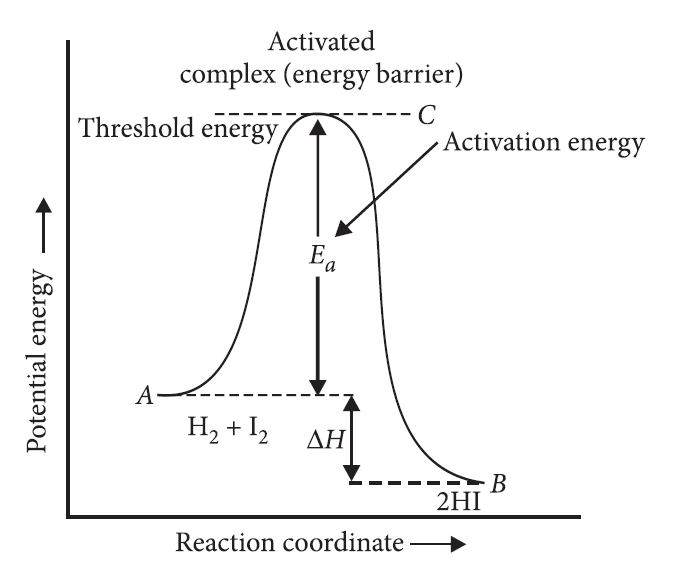
Effect of temperature on rate of reaction : For a chemical reaction with rise in temperature by 10° C, the rate constant is nearly doubled.
Arrhenius equation : k = ![]()
![]()
where, k = Rate constant,
A = Pre-exponential factor (frequency factor),
Ea = Activation energy,
T = Temperature
Activation energy : The minimum amount of energy required by reactant molecules to participate in a reaction is called activation energy (Ea).
Activation energy = Threshold energy – Average kinetic energy of reacting molecules
Define the rate constant.
Rate constant is the proportionality factor in the rate law expression for a chemical reaction. It is defined as the rate of a chemical reaction for which the concentration of each of the reacting species is unity.
Define the specific rate of reaction.
At a given temperature, rate is equal to the rate constant of reaction when concentration of the reactant in unity. Thus rate constant is also known as specific reaction rate.
In the case of two reactants, the reaction may be written as :
A + B → Products
![]()
where all the terms have their usual meaning. if CA = CB = 1 then r = k.
For a reaction A + B → P, the rate law is given by, r = k[A]1/2 [B]2
What is the order of this reaction?
Rate law, r = k[A]1/2 [B]2
Order of reaction is sum of the powers of concentration terms.
∴ Order of reaction = ![]()
If the rate constant of reaction is k = 3 × 10–4s–1, then identify the order of the reaction.
First order reaction.
Define ‘order of a reaction’.
It is defined as “the sum of the powers or exponents to which the concentration terms are
raised in the rate law expression.”
If rate = k[A]m [B]n, then order = m + n.
For a reaction : 2NH3(g) ![]() N2(g) + 3H2(g) ; Rate = k
N2(g) + 3H2(g) ; Rate = k
(i) Write the order and molecularity of this reaction.
(ii) Write the unit of k.
(i) The decomposition of gaseous ammonia on a hot platinum surface is a zero order reaction at high pressure.
In this reaction, platinum metal acts as a catalyst. At high pressure, the metal surface gets saturated with gas molecules. So, a further change in reaction conditions is unable to alter the amount of ammonia on the surface of the catalyst making rate of the reaction independent of its concentration.
However, two molecules of ammonia react to give products thus, the molecularity is two.
(ii) For a zero order reaction, unit of rate constant is mol L–1 s–1.
The half-life period for a zero order reaction is equal to
(a) ![]() (b)
(b) ![]()
(c) ![]() (d)
(d) ![]()
(where [R]0 is initial concentration of reactant and k is rate constant.)
Ans (d) ![]()
Define the half-life period of reaction (t½).
The time taken for half of the reaction to complete, i.e., the time in which the concentration of a reactant is reduced to half of its original value is called half-life period of the reaction.
t = t½ = When [R] = ![]()
Define: Pseudo first order reaction
Those reactions which are not truly of the first order but under certain conditions become reactions of the first order are called pseudo first order reactions.
What is the effect of adding a catalyst on
(1) activation energy (Ea), and
(2) Gibbs energy (ΔG) of a reaction?
(1) A catalyst lowers the activation energy (Ea) by providing an alternate pathway or reaction mechanism.
(2) Catalyst does not affect the Gibbs energy (ΔG) of a reaction.
With the help of a labelled diagram explain the role of activated complex in a reaction.