- Chemistry
- No Comment
Chemistry MCQ’s Quiz
Download
Q.1 The total number of atomic orbitals in fourth energy level of an atom is :
(1) 4
(2) 8
(3) 16
(4) 32
Q.2 The electrode potentials for
![]()
And ![]()
are + 0.15 V and + 0.50 V respectively. The value of ![]() will be :
will be :
(1) 0.150 V
(2) 0.500 V
(3) 0.325 V
(4) 0.650 V
Q.3 Mole fraction of the solute in a 1.00 molal aqueous solution is :
(1) 1.7700
(2) 0.1770
(3) 0.0177
(4) 0.0344
Q.4 By what factor does the average velocity of a gaseous molecule increase when the temperature (in Kelvin) is doubled ?
(1) 1.4
(2) 2.0
(3) 2.8
(4) 4.0
Q.5 A buffer solution is prepared in which the concentration of NH3 is 0.30 M and the concentration of NH4 + is 0.20 M. If the equilibrium constant, Kb for NH3 equals 1.8 × 10–5, what is the pH of this solution? (log 2.7 = 0.43)
(1) 8.73
(2) 9.08
(3) 9.43
(4) 11.72
Q.6 Two gases A and B having the same volume diffuse through a porous partition in 20 and 10 seconds respectively. The molecular mass of A is 49 u. Molecular mass of B will be :
(1) 25.00 u
(2) 50.00 u
(3) 12.25 u
(4) 6.50 u
Q.7 Which of the following is correct option for free expansion of an ideal gas under adiabatic condition ?
(1) q = 0, ΔT < 0, w ≠ 0
(2) q = 0, ΔT ≠ 0, w = 0
(3) q ≠ 0, ΔT = 0, w = 0
(4) q = 0, ΔT = 0, w = 0
Q.8 For the reaction N2(g) + O2(g) ⇌ 2NO(g), the equilibrium constant is K1. The equilibrium constant is K2 for the reaction 2NO(g) + O2(g) ⇌ 2NO2(g). What is K for the reaction
NO2(g) ⇌1/2 N2(g)+ O2(g)?
(1) ![]()
(2) ![]()
(3) ![]()
(4) ![]()
Q.9 If x is amount of adsorbate and m is amount of adsorbent, which of the following relations is not related to adsorption process ?
(1) ![]()
(2) ![]() = f(p) at constant T
= f(p) at constant T
(3) ![]() = f(T) at constant p
= f(T) at constant p
(4) p = f(T) at constant ![]()
Q.10 If the enthalpy change for the transition of liquid water to steam is 30 kJ mol–1 at 27ºC, the entropy change for the process would be :
(1) 100 J mol–1 K–1
(2) 10 J mol–1 K–1
(3) 1.0 J mol–1 K–1
(4) 0.1 J mol–1 K–1
Q.11 The Van’t Hoff factor i for a compound which undergoes dissociation in one solvent and association in other solvent is respectively :
(1) greater than one and greater than one
(2) less than one and greater than one
(3) less than one and less than one
(4) greater than one and less than one
4
Q.12 Standard electrode potential for Sn4+/Sn2+ couple is +0.15 V and that for the Cr3+/ Cr couple is –0.74 V. These two couples in their standard state are connected to make a cell. The cell potential will be :
(1) + 1.83 V
(2) + 1.19 V
(3) + 0.89 V
(4) + 0.18 V
3
Q.13 A gaseous mixture was prepared by taking equal mole of CO and N2. If the total pressure of the mixture was found 1 atmosphere, the partial pressure of the nitrogen (N2) in the mixture is :
(1) 1 atm
(2) 0.5 atm
(3) 0.8 atm
(4) 0.9 atm
2
Q.14 If the Eºcell for a given reaction has a negative value, then which of the following gives the correct relationships for the values of ΔGº and Keq ?
(1) ΔGº > 0; Keq < 1
(2) ΔG° > 0; Keq > 1
(3) ΔGº < 0; Keq > 1
(4) ΔG°< 0; Keq < 1
1
Q.15 The freezing point depression constant for water is –1.86ºCm–1. If 5.00 g Na2SO4 is dissolved in 45.0g H2O, the freezing point is changed by –3.82ºC. Calculate the Van’t Hoff factor for Na2SO4.
(1) 0.381
(2) 2.05
(3) 2.63
(4) 3.11
3
Q.16 The energies E1 and E2 of two radiations are 25 eV and 50 eV respectively. The relation between their wavelengths i.e. λ1 and λ2 will be:
(1) λ1 = ![]() λ2
λ2
(2) λ1 = λ2
(3) λ1 = 2λ2
(4) λ1 = 4λ2
3
Q.17 Standard electrode potential of three metals X, Y and Z are –1.2 V, + 0.5 V and –3.0 V respectively. The reducing power of these metals will be :
(1) X > Y > Z
(2) Y > Z > X
(3) Y > X > Z
(4) Z > X > Y
4
Q.18 Which one of the following statements for the order of a reaction is incorrect?
(1) Order of reaction is always whole number
(2) Order can be determined only experimentally
(3) Order is not influenced by stoichiometric coefficient of the reactants.
(4) Order of reaction is sum of power to the concentration terms of reactants to express the rate of reaction
1
Q.19 Enthalpy change for the reaction,
4H(g) → 2H2(g) is –869.6kJ The dissociation energy of H – H bond is
(1) +217.4 kJ
(2) –434.8 kJ
(3) –869.6 kJ
(4) +434.8 kJ
4
Q.20 If n = 6, the correct sequence for filling of electrons will be :
(1) ns → np(n – 1)d → (n – 2)f
(2) ns → (n – 2)f → (n – 1)d → np
(3) ns → (n – 1)d → (n – 2)f → np
(4) ns → (n – 2)f → np → (n – 1)d
2
Q.21 Which of the following compounds has the lowest melting point ?
(1) CaF2
(2) CaCl2
(3) CaBr2
(4) Cal2
4
Q.22 Which of the following pairs of metals is purified by van Arkel method ?
(1) Ni and Fe
(2) Ga and In
(3) Zr and Ti
(4) Ag and Au
3
Q.23 The correct order of increasing bond length of C– H, C – O, C – C and C = C is :
(1) C – H < C – O < C – C < C = C
(2) C – H < C = C < C – O < C – C
(3) C – C < C = C < C – O < C – H
(4) C – O < C – H < C – C < C = C
2
Q.24 Acidified K2Cr2O7 solution turns green when Na2SO3 is added to it. This is due to the formation of :
(1) CrSO4
(2) Cr2(SO4)3
(3) CrO42–
(4) Cr2(SO3)3
2
Q.25 For the four successive transition elements (Cr, Mn, Fe and Co), the stability of +2 oxidation state will be there in which of the following order?
(1) Cr > Mn > Co > Fe
(2) Mn > Fe > Cr > Co
(3) Fe > Mn > Co > Cr
(4) Co > Mn > Fe > Cr
(At nos. Cr = 24, Mn = 25, Fe = 26, Co = 27)
2
Q.26 Which of the two ions from the list given below that have the geometry that is explained by the same hybridization of orbitals, NO2−, NO3−, NH4−, SCN−?
(1) NO2– and NH2–
(2) NO2− and NO3−
(3) NO4+ and NO3−
(4) SCN– and NH2−
2
Q.27 Which of the following elements is present as the impurity to the maximum extent in the pig iron ?
(1) Phosphorus
(2) Manganese
(3) Carbon
(4) Silicon
3
Q.28 Which of the following is least likely to behave as Lewis base ?
(1) OH–
(2) H2O
(3) NH3
(4) BF3
4
Q.29 Which one of the following is present as an active ingredient in bleaching powder for bleaching action ?
(1) CaCl2
(2) CaOCl2
(3) Ca(OCl)2
(4) CaO2Cl
3
Q.30 The complex [Pt (Py) (NH3) Br Cl] will have how many geometrical isomers?
(1) 2
(2) 3
(3) 4
(4) 0
2
Q.31 Name the type of the structure of silicate in which one oxygen atom of [SiO4]4– is shared ?
(1) Three dimensional
(2) Linear chain silicate
(3) Sheet silicate
(4) Pyrosilicate
4
Q.32 The complexes [Co(NH3)6] [Cr(CN)6] and [Cr(NH3)6] [Co(CN)6] are the examples of which type of isomerism ?
(1) Geometrical isomerism
(2) Linkage isomerism
(3) Ionization isomerism
(4) Coordination isomerism
4
Q.33 The d-electron configurations of Cr2+, Mn2+, Fe2+ and Co2+ are d4, d5, d6 and d7 respectively. Which one of the following will exhibit minimum paramagnetic behaviour ?
(1) [Cr(H2O)6]2+
(2) [Mn(H2O)6]2+
(3) [Fe(H2O)6]2+
(4) [Co(H2O)6]2+
(At. Nos. Cr= 24, Mn= 25, Fe= 26, Co = 27)
4
Q.34 Of the following complex ions, which is diamagnetic in nature ?
(1) [CoF6]3
(2) [NiCl4]2–
(3) [Ni(CN)4]2–
(4) [CuCl4]2–
3
Q.35 Which of the following has the minimum bond length?
(1) O2
(2) O2+
(3) O2−
(4) O22−
2
Q.36 The value of ΔH for the reaction X2(g) + 4Y2(g) ⇌ 2XY4(g) is less than zero.
Formation of XY4(g) will be favoured at:
(1) High pressure and low temperature
(2) High temperature and high pressure
(3) Low pressure and low temperature
(4) High temperature and low pressure
1
Q.37 Of the following which one is classified as polyester polymer ?
(1) Nylon – 66
(2) Terylene
(3) Backelite
(4) Melamine
2
Q.38 What is the product obtained in the following reaction :
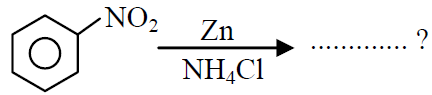
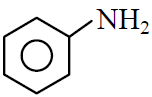
(1) 
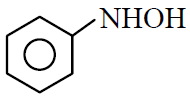
(2) 
(3) 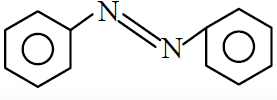
(4) 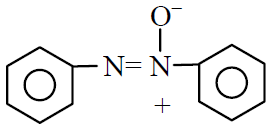
2
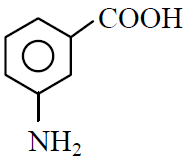
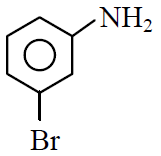
Q.39 In a set of reactions m-bromobenzoic acid gave a product D. Identify the product D.
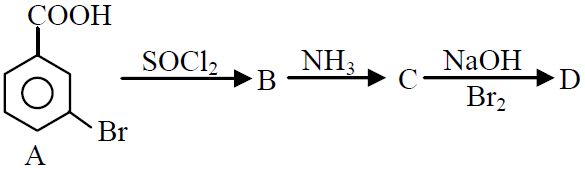
(1) 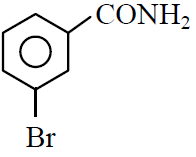
(2) 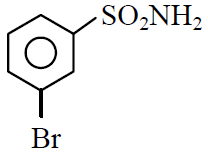
(3) 
(4) 
4
Q.40 In Dumas’ method of estimation of nitrogen 0.35 g of an organic compound gave 55 mL of nitrogen collected at 300 K temperature and 715 mm pressure. The percentage composition of nitrogen in the compound would be :
(Aqueous tension at 300 K = 15 mm)
(1) 14.45
(2) 15.45
(3) 16.45
(4) 17.45
3
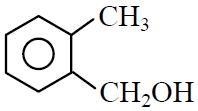
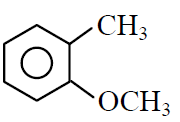
Q.41 Which one of the following is most reactive towards electrophilic reagent ?
(1) 
(2) 
(3) 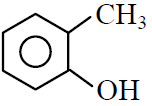
(4) 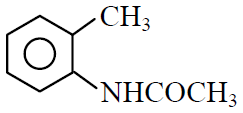
3
Q.42 Which one is a nucleophilic substitution reaction among the following ?
(1) CH3CHO + HCN → CH3CH(OH)CN
(2) 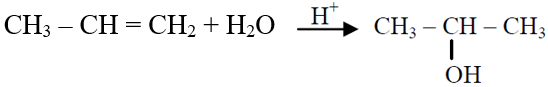
(3) 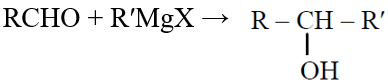
(4) 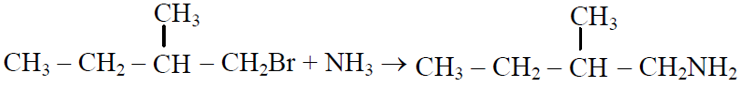
4
Q.43 Considering the state of hybridization of carbon atoms, find out the molecule among the following which is linear?
(1) CH3 – CH2 – CH2 – CH3
(2) CH3 – CH = CH – CH3
(3) CH3 – C ≡ C – CH3
(4) CH2 = CH – CH2 – C ≡ CH
3
Q.44 In the following reactions,
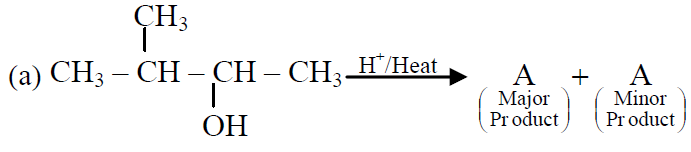
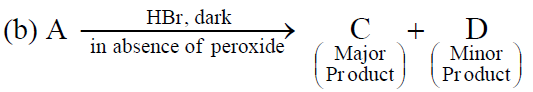
the major products (A) & (C) are respectively:
(1) 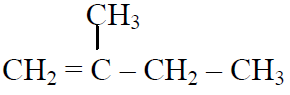 and
and 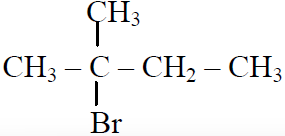
(2) 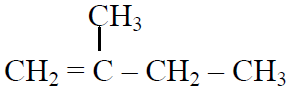 and
and 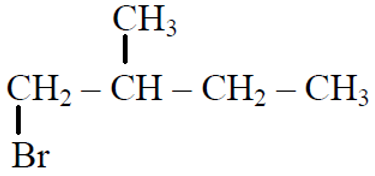
(3) 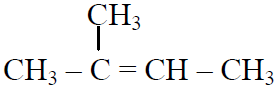 and
and 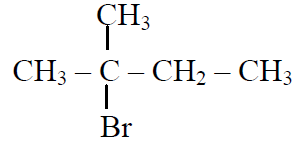
(4) 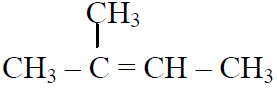 and
and 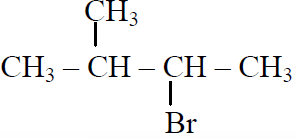
3
Q.45 The Lassaigne’s extract is boiled with conc. HNO3 while testing for halogens. By doing so it:
(1) increases the concentration of NO3– ions
(2) decomposes Na2S and NaCN, if formed
(3) helps in the precipitation of AgCl
(4) increases the solubility product of AgCl
2
Q.46 The correct IUPAC name of the compound 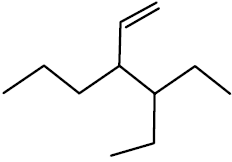
is
(1) 3-(1-ethyl propyl) hex-1 ene
(2) 4-Ethyl-3-propyl hex-1-ene
(3) 3-Ethyl-4-ethenyl heptane
(4) 3-Ethyl-4-propyl hex-5-ene
2
Q.47 Clemmensen reduction of a ketone is carried out in the presence of which of the following ?
(1) H2 and Pt as catalyst
(2) Glycol with KOH
(3) Zn-Hg with HCl
(4) Li Al H4
3
Q.48 Which one of the following is employed as Antihistamine ?
(1) Omeprazole
(2) Chloramphenicol
(3) Diphenyl hydramine
(4) Norothindrone
3
Q.49 Which one of the following statements is not true regarding (+) Lactose ?
(1) (+) Lactose, C12H22O11 contains 8-OH groups
(2) On hydrolysis (+) Lactose gives equal amount of D(+) glucose and D(+) galactose
(3) (+) Lactose is a β-glycoside formed by the union of a molecule of D(+) glucose and a molecule of D(+) galactose
(4) (+) Lactose is a reducing sugar and does not exhibit mutarotation
4
Q.50 Which one of the following statement is not true?
(1) Oxide of sulphur, nitrogen and carbon are the most widespread air pollutant
(2) pH of drinking water should be between 5.5 – 9.5
(3) Concentration of DO below 6 ppm is good for the growth of fish
(4) Clean water would have a BOD value of less than 5ppm
3