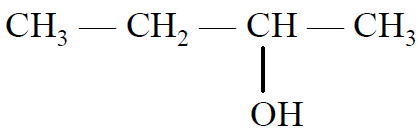ALCOHOL


1- INTRODUCTION
Their general formula is CnH2n+1OH or CnH2n+2O.
These are of following types, depending upon the no. of OH groups.
(i) Monohydric alcohol:-
Contains one -OH group only, eg. C2H5OH
(ii) Dihydric alcohol :-
Contains two -OH groups- eg. glycol
(iii) Trihydric alcohol:-
Contains three -OH groups eg. glycerol
(iv) Polyhydric alcohol :-
Contains more than three -OH groups- eg, sorbitol, mannitol.
2- METHODS OF PREPARATION
2-1 From Alkyl halides:-
Alkyl halides reacts with aq. KOH/aq. AgOH or H2Oand forms alcohol-
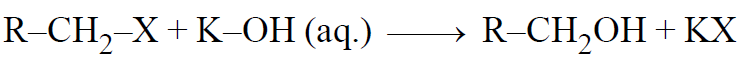
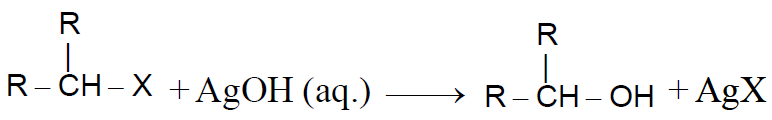
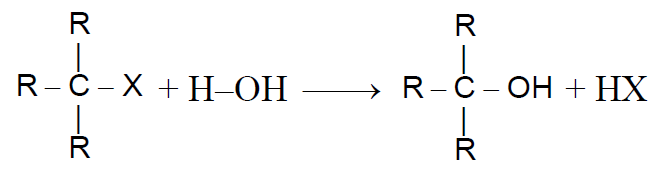
2-2 From Alkenes :
2-2-1 Hydration –Alkenes are catalytically hydrated by dilute mineral acid solution-
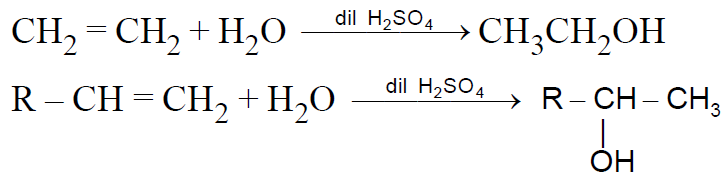
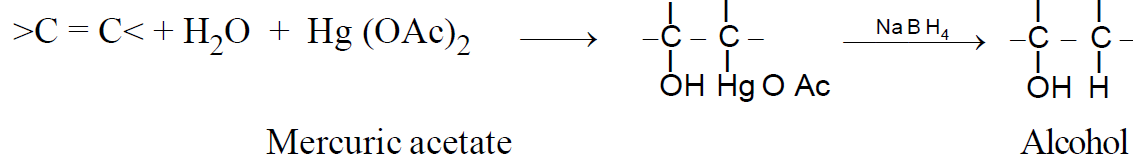
2-2-2 Oxymercuration – demercuration :
Alkenes react with mercuric acetate in the presence of water to give hydroxy mercurial compounds, which on reduction yield alcohols- (Markovnikov addition)
2-2-3 Hydroboration -Oxidation :
(Anti-Markownikov orientation)
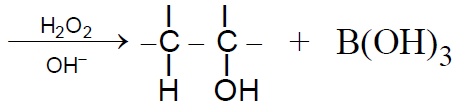
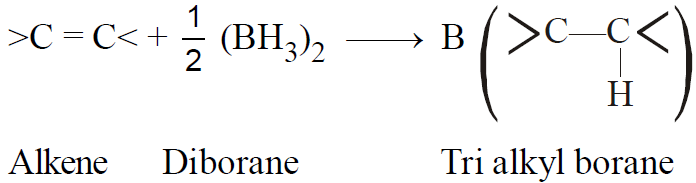
2-3 ByReduction ofCarbonyl compounds :-
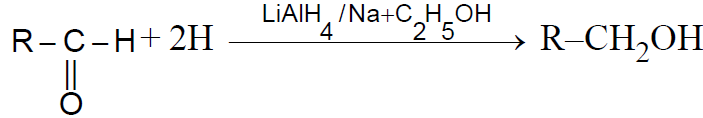
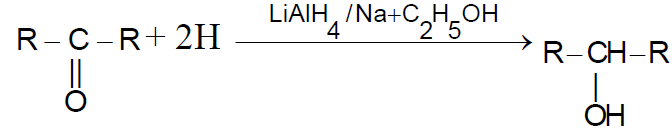
Note :
(i) We cannot obtain 3º alcohol from this method
(ii) If we use NaH as reductant then the process is called as ‘Darzen’s process‘-
From Primary amines :-
R-NH2 + HNO2 ![]() R-OH + N2 + H2O
R-OH + N2 + H2O
But it is not a good method for preparation of alcohol because a number of byproducts are formed in this reaction like alkyl chloride, alkyl nitrite, alkene and ether.
Note : In this reaction if we take ethyl amine then main product will be ethanol while if we take methyl amine, then main product will be dimethyl ether
PHYSICALPROPERTIES
(a) Alcohols are colourless with specific smell liquid-They are soluble is water due to H-bonding.
These are partially soluble in organic solvents.
(b) They are liquid in nature up to 12-carbon.
(c) Melting point and Boiling point ∝ molecular mass ∝ 1 / No. of branches
(d) Boiling point of alcohols are higher than equivalent ethers. It is due to H-bonding.
(e) Alcohols are poisonous in nature also-Poisonous character increase with increment in molecular weight or branching. Ethanol is exception, which is non-poisonous in nature. It is most useful organic solvent.
(f) Methanol causes blindness.
(g) Isopropyl alcohol is called as rubbing alcohol.
(h) Cholestrol is also an example of complex alcohol which is called notorious alcohol because it causes heart attack.
(i) Viscous nature of alcohol is directly proportional to H-bonding or number of -OH groups. That is why we can say alcohol is less viscous than glycerol & manitol is more viscous than glycerol.
(j) Ethanol is liquid while glucose is solid. It is due to more H-bonding in glucose.
CHEMICAL PROPERTIES
Chemical reactions of alcohols are classified in the following three types :-
(i) Reaction of H atom of -OH group of Alcohols
(ii) Reaction of OH group of Alcohols
(iii) General reaction of Alcohols.
Reaction of H atom of -OH group of Alcohols :
These are the reactions in which alcohol shows acidic character.
Reaction with Na :
![]() 2R-O-H + Na 2R – O – Na + H2↑
2R-O-H + Na 2R – O – Na + H2↑
The acidic order of alcohols is
MeOH > 1º > 2º > 3º
Esterification /Reaction with carboxylic acid:-
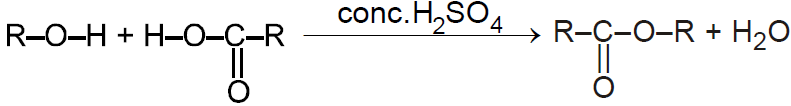
Reaction with Acid derivatives :
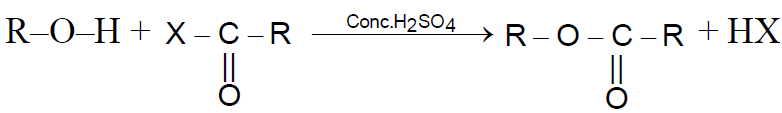
Reaction with Ketene :-
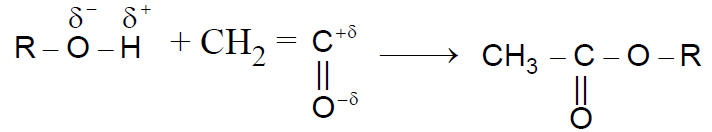
Reaction with Isocyanic Acid :-
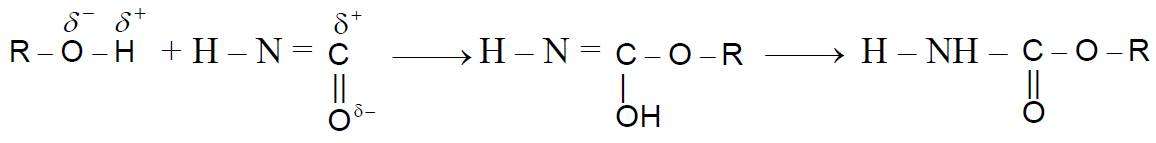
Reaction of -OH group of Alcohols :-
Reaction with dry HX (Grove’s Process) :
![]() R-OH + HX R – X + H2O
R-OH + HX R – X + H2O
Reaction with PCl5 :
R-OH + PCl5 ![]() R-Cl + POCl3 +HCl
R-Cl + POCl3 +HCl
Reaction with PCl3 :
3R-OH + PCl3 ![]() 3R-Cl + H3PO3
3R-Cl + H3PO3
Reactionwith SOCl2 (Darzen reaction): –
R-OH +SOCl2 ![]() R-Cl +SO2 +HCl
R-Cl +SO2 +HCl
Reaction with ammonia :
 R – OH+ NH3 RNH2 + H2O
R – OH+ NH3 RNH2 + H2O
Reaction with HNO3 :
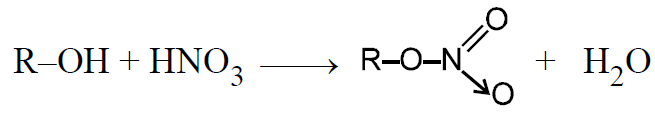
DIFFERENCE BETWEEN PRIMARY, SECONDARY & TERTIARY ALCOHOLS
By Oxidation Reaction :-
Primary alcohol gives aldehyde on oxidation, secondary alcohol gives ketone and tertiary alcohols are resistant to oxidation-
By Catalytical Oxidation / Dehydrogenation :
Primary alcohol gives aldehyde on oxidation, secondary alcohol gives ketone and tertiary alcohol gives alkene (dehydration takes place in this condition to tertiary alcohols.)
Lucas Test :-
A mixture of (anhydrous ZnCl2 +Conc-H2SO4) is called as Lucas Reagent.
(i) 3º alcohol gives white ppt. with lucas reagent in 2-3 seconds only.
(ii) 2º alcohol takes 9 – 10 minutes.
(iii) 1º alcohol does not gives white ppt. at room temperature.
Victor Meyer Test : –
This test is also known as RBW( Red, Blue, White) test-
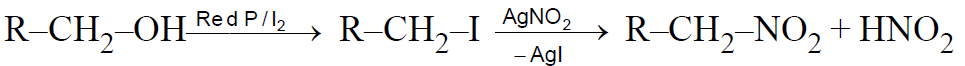

MCQ
Q- Which of the following is produced when an aqueous solution of butan-2-ol is refluxed with dilute acidic KMnO4?
(A) butanol (B) butanoic acid
(C) potassiumbutanoate (D) butanone
Ans D
Q- Chlorine reacts with ethanol to give
- Ethyl chloride (B) chloroform (C) chloral (D) acetaldehyde
Ans C
Q- Ethanol is heated with concentratedH2SO4. The product formed is
(A) 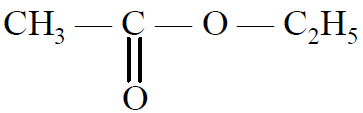 (B) C2H6
(B) C2H6
(C) C2H4 (D) C2H2
Ans C
Q- Which of the following has the highest boiling point?
(B) 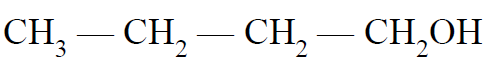
(C) 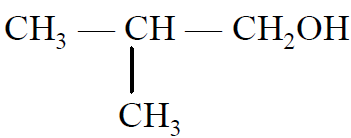
(D) 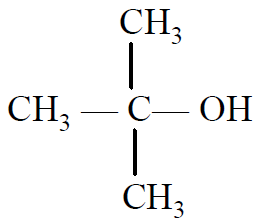
Ans B
Q- Lucas test is used to determine the type of
(A) amines (B) alcohols (C) acids (D) phenols
Ans B
Q- Separation of a higher phenol and an aromatic carboxylic acid can be easily carried out by
(A) NaOH (B) Na2CO3 (C) lime (D) NaHCO3
Ans D
Q- The reaction between sodiumethoxide and bromoethane yields
(A)methyl ethyl ether (B) dimethyl ether
(C) diethyl ether (D) propane
Ans C
Q- The hydroboration oxidation of 2-methyl propene yields-
(A) 1º alcohol (B) 2º alcohol (C) 3º alcohol (D) None
Ans A
Q- The alkaline hydrolysis of esters is known as:
(A) Hydration (B) Esterification
(C) Dehydration (D) Saponification
Ans D
Q- Which of the following reactions of an alcohol does not involve O – H bond breaking :
(A) Reaction with alkali metals (B) Reaction with an acyl chloride
(C) Reaction with sulphonyl chloride (D) Reaction with conc- sulphuric acid-
Ans D
Q- Replacement of -OH group in alcohol by -Cl cannot be carried out with-
(A) PCl5 (B) SO2Cl2 (C) PCl3 (D) SOCl2
Ans B
17- Which of the following alcohols does not give a red colour in Victor Meyer test-
(A) Isobutyl alcohol (B) Isoamyl alcohol
(C) Diethyl carbinol (D) Phenylcarbinol
Ans C
Q- Absolute alcohol contains-
(A) 40% H2O (B) 10% H2O (C) 5% H2O (D) 100% C2H5OH
Ans D
Q- Proof spirit contains about-
(A) 40% alcohol by weight (B) 50% alcohol by weight
(C) 25% alcohol by weight (D) 10% alcohol by weight
Ans B
Q- Reaction of alcohol does not show cleavage of R-O linkage-
(A) ROH + PCl5 (B) ROH + SOCl2 (C) ROH + HCl (D) ROH + Na
Ans D
Q- An organic compound dissolved in dry benzene, evolved hydrogen on treatment with sodium- It is-
(A) A ketone (B) An aldehyde (C) A tertiary amine (D) An alcohol
Ans D
Q- Alkyl chloride is formed when alcohol is treated with HCl in presence of anhydrous ZnCl2. The order of reactivity with respect to alcohol is :
(A) 3º > 2º > 1º (B) 1º > 2º > 3º (C) 2º > 1º > 3º (D) 1º > 3º > 2º
Ans A
Q- The increasing order of boiling points of 1º, 2º, 3º alcohol is –
(A) 1º > 2º > 3º (B) 3º > 2º > 1º
(C) 2º > 1º > 3º (D) None
Ans A
Q- Hydrogen bonding is possible in-
(A) Ethers (B) Hydrocarbons (C) Alkanes (D)Alcohols
Ans D
Q- The solubility of lower alcohols in water is due to –
(A) Formation of hydrogen bond between alcohol and water molecules
(B) Hydrophobic nature of alcohol
(C) Increases in boiling points
(D) None of these
Ans A
Q- A compound ‘A’ reacts with sodium metal and also undergoes iodoform reaction- ‘A’ is
(A) phenol (B)methanol (C) n-propanol (D) iso-propanol
Ans D
Q- Which of the following ethers is cleaved even byHCl at roomtemperature?
(A) C6H5OCH2CH3 (B) CH3CH2OCH2CH3
(C) (CH3)3COCH2CH3 (D) (CH3)3COC(CH3)3
Ans D
Q- Anhydride of alcohol is-
(A) Ether (B)Aldehyde
(C)Alkanoic anhydride (D)Alkoxides
Ans A
Q- When CH2=CH-COOH is reduced with LiAlH4, the compound obtained will be – [AIEEE-2003]
(A) CH3-CH2-CH2OH (B) CH3-CH2-CHO
(C) CH3-CH2-COOH (D) CH2=CH-CH2OH
Ans D
Q- Among the following compoundswhich can be dehydrated veryeasilyis – [AIEEE-2004]
(A) CH3CH2CH2CH2CH2OH (B) CH3 CH2 CH2 CH3
(C) CH3CH2 CH3 (D) CH3 CH2 CH2 CH2OH
Ans D
Q- For which of the following parameters the structural isomers C2H5OH and CH3OCH3 would be expected to have the same values? (Assume ideal behaviour) [AIEEE-2004]
(A)Heat of vaporization
(B)Vapour pressure at the same temperature
(C)Boiling points
(D)Gaseous densities at the same temperature and pressure
Ans D
Q- A liquid was mixed with ethanol and a drop of concentrated H2SO4 was added. A compound with a fruity smell was formed. The liquid was [AIEEE 2009]
(A) HCHO (B) CH3COCH3 (C) CH3COOH (D) CH3OH
Ans C
Q- When phenol is treated with bromine water in excess, it gives
(A) m-bromophenol (B) o-and p-bromophenol
(C) 2, 4-dibromophenol (D) 2, 4, 6-tribromophenol
Ans D
Q- The compound that does not change the orange colour of chromic acid to blue green is
(A) 2° alcohol (B) 1° alcohol (C) 3° alcohol (D) none of the above
Ans C
Q- HBr reacts the fastest with
(A) 2-methyl propan-2-ol (B) propan-1-ol
(C) propan-2-ol (D) 2-methyl propan-1-ol
Ans A
Q- A compound ‘X’ on oxidation gave ‘B’ and then again on oxidation gave an acid. After the first oxidation, it reacted with ammoniacal silver nitrate to produce a black precipitate. ‘X’ is
(A) a primary alcohol (B) a tertiary alcohol (C) acetaldehyde (D) acetone
Ans A
Q- R-CH2-CH2OH can be converted into RCH2CH2COOH. The correct sequence of reagent is
(A) P Br3, KCN, H+ (B) P Br3, KCN, H2
(C) KCN, H+ (D) HCN, PBr3, H+
Ans A
Q- Which of the following has a higher pH?
(A) phenol (B) o-cresol (C) p-nitrophenol (D) glycerol
Ans D
Q- The reaction of anisolewithHI leads to the formation of
(A) phenol andmethanol (B) phenol andmethyl iodide
(C) iodobenzene andmethanol (D) iodobenzene andmethyl iodide
Ans B
Q- Methyl-tert-butyl ether on heatingwithHI gives
(A) CH3I + (CH3)3COH (B) CH3OH + (CH3)3CCI
(C) CH3I + (CH3)3CI (D) (CH3)3CCI + CH3OH
Ans C
Q- Which of the following compounds is resistant to nucleophilic attack by hydroxyl ions?
(A)methyl acetate (B) acetonitrile (C) dimethyl ether (D) acetamide
Ans C
Q- Diethyl ether absorbs oxygen to form
(A) a red-coloured sweet-smelling compound (B) acetic acid
(C) ether suboxide (D) ether peroxide
Ans D
Q- On boilingwith concentratedHBr, phenyl ethyl etherwill yield
(A) phenol and ethyl bromide (B) bromobenzene and ethanol
(C) phenol and ethane (D) bromobenzene
Ans A
Q- Which of the following alcohols is least soluble in water ?
(A) n-Butyl alcohol (B) iso-Butyl alcohol (C) tert-Butyl alcohol (D) sec-Butyl alcohol
Ans A
Q- 2-Bromopentane is heated with potassium ethoxide in ethanol-The major product obtained is-
(A) 2-ethoxypentane (B) pentene-1 (C) cis-pentene-2 (D) trans-pentene-2
Ans D
Q- Which of the following reactions of alkanols does not involve C – O bond breaking :
(A) CH3CH2OH + SOCl2 (B) CH3CH(OH)CH3 + PBr3
(C) CH3CH2OH + CH3COOH (D) ROH + HX
Ans D
Q- Reactivity of alcohols with HCl is in the order of :
(A) Tert butyl alcohol > sec- butyl alcohol > primary butyl alcohol
(B) Primary butyl alcohol > sec- butyl alcohol > tert butyl alcohol
(C) Sec- butyl alcohol > tert butyl alcohol >primary butyl alcohol
(D) Sec- butyl alcohol > primary butyl alcohol > tert butyl alcohol
Ans A
Q- Ethyl alcohol is less acidic than phenol because :
(A) The phenoxide ion is more resonance stabilized than phenol
(B) There is more hydrogen bonding in phenol than in ethyl alcohol
(C) The ethoxide ion is less resonance stabilized than ethyl alcohol
(D) Phenol has a higher b-p- than ethyl alcohol
Ans A
Q- The compound obtained by the acidolysis of ether [dil- H2SO4] gives the following test :
(A) Yellow precipitate with NaOH and I2
(B) Smell of formaline with hot copper wire
(C) Smell of oil of winter-green with salicylic acid and H2SO4
(D)Acid obtained by oxidation decolourises KMnO4
Ans A
Q- 2-Bromoethanol reacts with magnesium to from :
(A) HOCH2CH2MgBr (B) CH3 – CH2OH
(C) CH3CH2MgBr (D) BrCH2-CH2MgBr
Ans B
Q- Methylethylketone can be obtained by the oxidation of :
(A) 2-Butanol (B) 2-Propanol (C) 1-Butanol (D) Tert-butyl alcohol
Ans A
Q- An alcohol (ROH) reacts with an acid to form initially :
(A) RCH2 -H2 (B) R+ (C) R-H2 (D)An alkene
Ans D
Q- will have the minimum value of pKa when X is :
(A) F (B) Cl (C) Br (D) NO2
Ans D
Q- The oxidation of a secondary alkanol with Cr (VI) leads to the formation of :
(A) An alkanone and Cr (II) (B) An aldehyde and Cr (III)
(C) An alkanone and Cr (III) (D) An aldehyde and Cr (II)
Ans C