- Chemistry
- No Comment
Chemistry MCQ Quiz
Download
Download
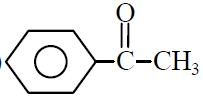
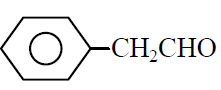
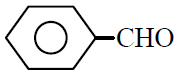
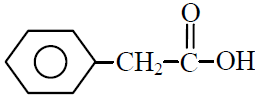
Q.1 Phenyl acetylene reacts with dil. H2SO4 in presence of HgSO4 gives :
(1) 
(2) 
(3) 
(4) 
1
Q.2 According to hardy Schultze law the order of coagulation power of cations will be :
(1) Na+ > Ba+2 > Al+3 (2) Al+3 > Ba+2 > Na+
(3) Ba+2 > Al+3 > Na+ (4) Al+3 > Na+ > Ba+2
2
Q.3 Which of the following compound gives p-cresol with p-methyl diazonium chloride :
(1) H2O (2) H3PO2
(3) HCOOH (4) C6H5OH
1
Q.4 Mole ratio of H2 and O2 gas is 8 : 1 what will be the ratio of wt. :
(1) 1 : 1 (2) 2 : 1
(3) 4 : 1 (4) 1 : 2
4
Q.5 Ionization energy of second orbit of Li+2 will be :
(1) 122.4 eV (2) 40.8 eV
(3) 30.6 eV (4) 13.6 eV
3
Q.6 Which of the following electronic configuration will have maximum I.P. difference between II and III I.P. :
(1) 1s2 2s2 2p6 3s1 (2) 1s2 2s2 2p6 3s2
(3) 1s2 2s2 2p6 (4) 1s2 2s2 2p5
2
Q.7 The concentration of a solution is changed from 0.2 to 0.4, then what will be rate and rate constant. The reaction is of first order and rate constant is K = 1 × 10–6 :
(1) 2 ×10–2 ; 1 × 10–6 (2) 1 ×10–2 ; 1 × 106
(3) 4 ×10–2 ; 1 × 10–6 (4) 2 ×10–3 ; 1 × 10–3
3
Q.8 Half life of a radioactive sample is 4 days. After 16 days how much quantity of matter remain undecayed :
(1) ![]()
(2) ![]()
(3) ![]()
(4) ![]()
3
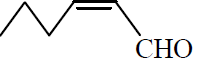
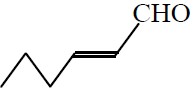
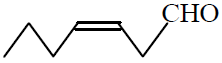
Q. 9 Structure of trans 2-hexanal is :
(1) 
(2) 
(3) 
(4) None of the above
2
Q.10 Which of the following gives ethyl benzene with phenyl methyl ketone :
(1) Zn–Hg+HCl (2) LiAlH4
(3) KMnO4 (4) None of the above
1
Q.11 Acetaldehyde reacts with semicarbazide product will be :
(1) CH3CH = NNH–CO–NH2
(2) CH3CH = NCONHNH2
(3) CH3CH = NHNH2
(4) 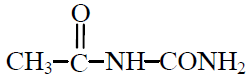
1
Q.12 Cynohydrin of the following compound on hydrolysis gives optically active product :
(1) HCHO (2) CH3CHO
(3) CH3COCH3 (4) All of the above
2
Q.13 Which of the following is a chiral compound :
(1) 2-methyl pentanoic acid
(2) 3-methyl pentanoic acid
(3) 4-methyl pentanoic acid
(4) None of these
1,2
Q.14 Compound ‘A’ on chlorination gives compound ‘B’. ‘B’ reacts with alc. KOH gives gas ‘C’, which decolourises Baeyer reagent and ozonolysis of
compound ‘C’ gives only HCHO compound ‘A’ is :
(1) C2H6 (2) C2H4
(3) C4H10 (4) C2H5Cl
1
Q.15 Monomer of natural rubber is :
(1) 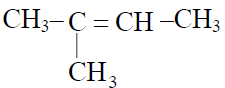
(2) CH3 –CH=CH–CH3
(3)
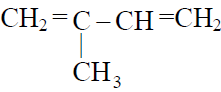
(4) 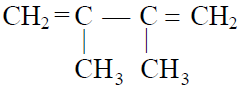
3
Q.16 Which of the following compound contain zero oxidation state of Fe :
(1) [Fe(CN)6]–4
(2) [Fe(CN)6]–3
(3) Fe(CO)5
(4) All the above
3
Q.17 A compound contain C, H and O. If C = 40% and H = 6.62% then empirical formula of compound will be :
(1) CH2O (2) CH4O
(3) CH4O2 (4) CHO
1
Q.18 [Cu(NH3)4]+2 reacts with HNO3 in excess of water gives :
(1) Cu(OH)2 (2) Cu(NO3)2
(3) Cu(H2O)–2 (4) None of the above
2
Q.19 Cr in [Cr(NH3)6] Br3 has number of unpaired electron :
(1) 4 (2) 3
(3) 1 (4) 2
2
Q.20 Sucrose on hydrolysis gives :
(1) L(+) Glucose + D(+) Fructose
(2) L(–) Glucose + L(–) Fructose
(3) D(+) Glucose + D(–) Fructose
(4) D(+) Glucose + L(–) Fructose
3
Q.21 Order of acidic strength of the following compound will be :
(A) 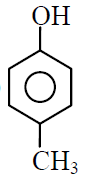
(B) C6H5OH
(C) 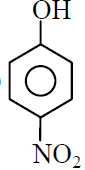
(D) 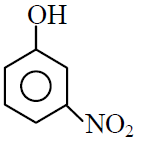
(1) C > D > B > A
(2) D > C > B > A
(3) A > B > C > D
(4) B > A > C > D
1
Q.22 Which of the following comp. is coloured and has unpaired electron :
(1) CuF2 (2) K2Cr2O2
(3) KMnO4 (4) K4[Fe(CN)6]
1
Q.23 Which of the following does not reduce Fehling solution :
(1) Glucose (2) Fructose
(3) Sucrose (4) Maltose
3
Q.24 O.N. of P in pyrophosphoric acid is :
(1) + 5 (2) + 2
(3) + 3 (4) + 4
1
Q.25 Which of the following example behave as a lewis acid BF3, SnCl2, SnCl4:
(1) Stenus chloride, stenic chloride
(2) BF3, stenus chloride
(3) Only BF3
(4) BF3, stenus chloride, stenic chloride
4
Q.26 In which of the following comp. H atom is directly linked with phosphorus :
(1) H3PO2 (2) H3PO3
(3) H3PO4 (4) H4P2O2
1,2
Q.27 a Zn + b NO3− + cH+ → dNH4+ + e H2O + f Zn+2 a, b, c, d, e and f are :
|
a |
b |
c |
d |
e |
f | |
|
(1) |
2 |
4 |
6 |
8 |
4 |
2 |
|
(2) |
1 |
4 |
10 |
3 |
1 |
4 |
|
(3) |
4 |
1 |
10 |
1 |
3 |
4 |
|
(4) |
10 |
4 |
1 |
3 |
4 |
2 |
3
Q.28 Determine the value of E0 cell for the following reaction :
Cu+2 + Sn+2 → Cu + Sn+4
Equilibrium constant is 106
Cu++ + Sn++ → Cu + Sn+4
(1) 0.1223 (2) 0.01223
(3) 0.2153 (4) 1.223
1
Q.29 What will be the H+ con when 4 gm NaOH dissolved in 1000 ml. of water:
(1) 10–1 (2) 10–13
(3) 10–4 (4) 10–10
2
Q.30 What is true for a cyclic process :
(1) W = 0 (2) ΔE = 0
(3) ΔH = 0 (4) ΔE ≠ 0
2,3
Q.31 Increasing order of bond length is :
(1) NO– < NO < NO+ < O2−
(2) O2− < NO < NO– < NO+
(3) O2– < NO– < NO < NO+
(4) NO+ < NO < NO– < O2−
4
Q.32 A system is expanded under adiabatic process :
(1) Temp. increase (2) ΔE decreases
(3) ΔE increases (4) None of these
2
Q.33 Which of the following is true for a reaction in which all the reactant & product are liquids :
(1) ΔH = ΔE
(2) ΔH = ΔW
(3) ΔH > ΔE
(4) None of the above
1
Q.34 Clemenson’s reaction is :
(1) 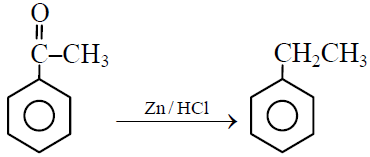
(2) ![]()
(3) ![]()
(4) All the above
1
Q.35 Which of the following reaction gives by isocyanide :
(1) Rimer Tieman reaction
(2) Carbyl amine reaction
(3) Hoffmann bromamide reaction
(4) None of the above
2
Q.36 In a gaseous mixture which of NO2, CO2 and N2O gases have same rate of diffusion :
(1) NO2, CO2 (2) CO2, N2O
(3) NO2, N2O (4) All
2
Q.37 Compound ‘A’ in acidic medium does not give ppt with H2S but in NH4OH medium gives a ppt comp. ‘A’ is :
(1) FeCl3 (2) AlCl3
(3) ZnCl2 (4) SnCl2
3
Q.38 FeCr2O2 reacts with Na2CO3 gives the product :
(1) Na2CrO4 (2) Na2Cr2O2
(3) Fe3O4 (4) FeO
1
Q.39 A compound BA2 has Ksp = 4 × 10–12 solubility of this comp. will be :
(1) 10–3 (2) 10–4 (3) 10–5 (4) 10–6
2
Q.40 H2O2 on oxidation gives :
(1) O–2 (2) OH– (3) O2− (4) O2
4
Q.41 What is false for mole fraction :
(1) x < 1 (2) – 2 ≤ x ≤ 2
(3) 0 ≤ x ≤ 1 (4) Always non-negative
2
Q.42 MgO and NaCl has similar structure. In MgO magnesium is surrounded by how many oxygen atoms :
(1) 2 (2) 4 (3) 6 (4) 1
3
Q.43 General behaviour of O3 is :
(1) Gives electrons (2) Gives O2
(3) Reaction with H2 (4) Accept electrons
2
Q.44 How many ATP will be formed by oxidation of 1 mole glucose :
(1) 36 (2) 40
(3) 24 (4) 32
1
Q.45 400 ml gas at 500 torr and 666.6 ml gas at 600 torr taken in a container of 3 litre then the total pressure of mixture :
(1) 200 torr (2) 400 torr
(3) 600 torr (4) 50 torr
1
Q.46 Which of the following is steroid harmones :
(1) Progesterone (2) Cholesterole
(3) ACTH (4) Adrenaline
1
Q.47 The dipole moment of compound AB is 10.92 D and that of compound CD is 12.45 D. The bond length AB is 2.22 A0 and that of CD is 2.56 A0 then for these compound true statement is :
(1) More ionic nature in AB
(2) More ionic nature in CD
(3) Equal in both
(4) Not predicted
2
Q.48 The bombarment of α-particle on 2N14, emits proton then new atom will be :
(1) 8O12 (2) 8O16
(3) 6C14 (4) Ne
1
Q.49 Half life of a substance is 22 days then its decay constant will be :
(1) 0.9 (2) 0.09
(3) 0.009 (4) 0.013
3