Experiment No. 1
Exercise: Find out the concentration of potassium permanganate in gram per litre. For this purpose you are provided with a standard solution of crystalline ferrous ammonium sulphate of molarity M/30
Theory: Titration between potassium permanganate and ferrous ammonium sulphate is a redox titration. KMnO4 in presence of dil. H2SO4 oxidises ferrous ammonium sulphate to ferric sulphate [Fe2(SO4)3] and itself is reduced to manganese sulphate (MnSO4).
(1) Molecular equation
2KMnO4 + 3H2SO4 → K2SO4 + 2MnSO4 + 3H2O+5[0]
[2FeSO4(NH4)2SO4.6H2O + H2SO4 + [O] → Fe2(SO4)3 + 2(NH4)2SO4 + 13H20] × 5
2KMnO4+10FeSO4 (NH4)2SO4.6H2O+8H2SO4→K2SO4 + 2MnSO4 +5Fe2(SO4)3 + 10(NH4)2SO4 +68H2O
(ii) Ionic Equation :
![]()
![]()
![]()
Indicator :- KMnO4 is self indicator.
Observation table:
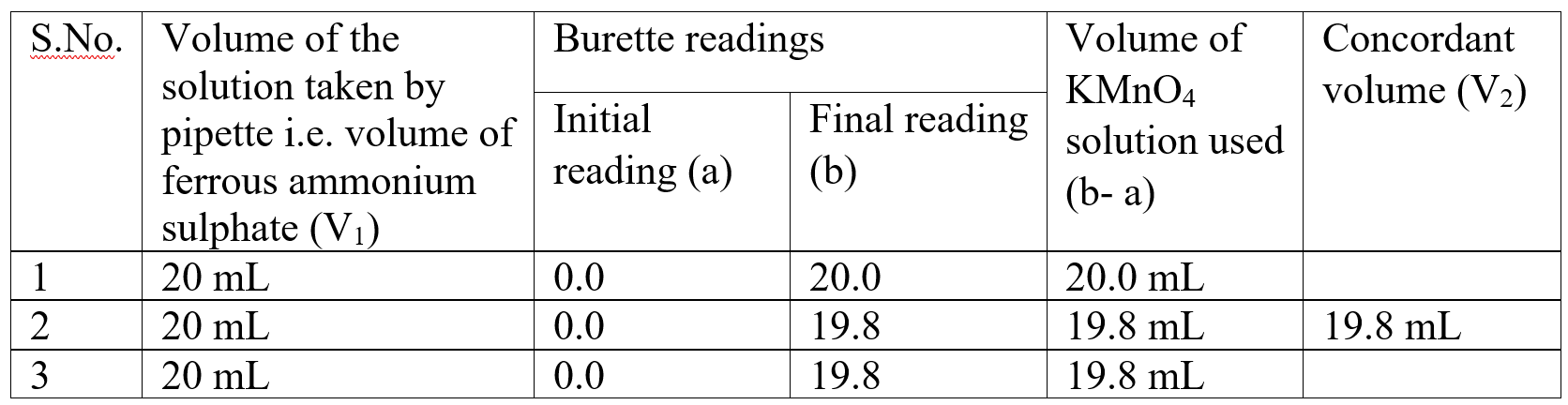
Calculations: (A) Calculation of Molarity of KMnO4
It is clear from the molecular equation of the reaction between KMnO4 and ferrous ammonium sulphate that 2 moles of KMnO4 oxidise 10 moles of ferrous ammonium sulphate. i.e. 10 moles of Ferrous ammonium sulphate = 2 mol KMnO4
![]()
Or M1V1 = 5 M2V2
M1 = Molarity of ferrous ammonium sulphate solution = M/30
V1 = Volume of Ferrous ammonium sulphate solution = 20 mL
M2 = Molarity of KMnO4 solution = ?
V2 = Volume of KMnO4 solution = = 19.8 mL
M2 = ![]()
M2 = ![]()
M2 = ![]() M
M
(B) Calculation of Strength is gms/litre of KMnO4 solution:
Strength is gm/litre = Molarity × Molar mass
![]()
= 1.0640 gm per litre
Result: The strength of the given KMnO4 solution is 1.0640 gL-1
Experiment No. 2
Exercise: Find out the molarity of potassium permanganate solution. For this purpose you are provided with a standard solution of crystalline ferrous ammonium sulphate containing 13.0666 grams of the salt per litre.
Theory: Titration between potassium permanganate and ferrous ammonium sulphate is a redox titration. KMnO4 in presence of dil. H2SO4 oxidises ferrous ammonium sulphate to ferric sulphate [Fe2(SO4)3] and itself is reduced to manganese sulphate (MnSO4).
(1) Molecular equation
2KMnO4 + 3H2SO4 → K2SO4 + 2MnSO4 + 3H2O+5[0]
[2FeSO4(NH4)2SO4.6H2O + H2SO4 + [O] → Fe2(SO4)3 + 2(NH4)2SO4 + 13H20] × 5
2KMnO4+10FeSO4 (NH4)2SO4.6H2O+8H2SO4→K2SO4 + 2MnSO4 +5Fe2(SO4)3 + 10(NH4)2SO4 +68H2O
(ii) Ionic Equation :
![]()
![]()
![]()
Indicator :- KMnO4 is self indicator.
Observation table:
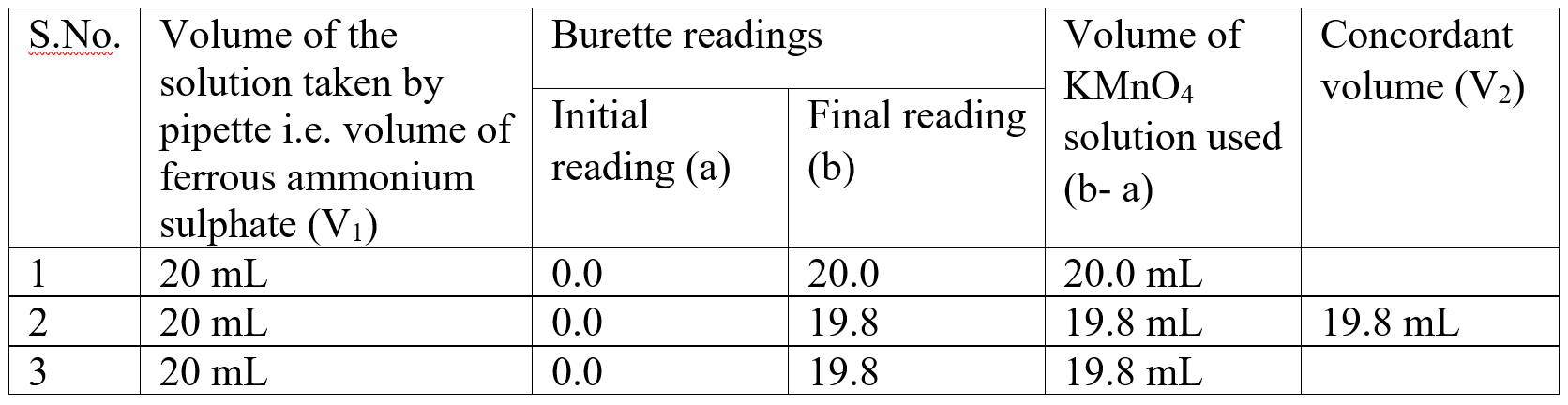
Calculations: (i)Molarity of Ferrous ammonium sulphate :
Weight of Ferrous Ammonium sulphate = 13.0666 grams (given)
Volume of Ferrous Ammonium sulphate solution = 1000 mL
Molar mass of Ferrous Ammonium sulphate (FAS) = 392.12
Molarity of solution M1 = ![]()
Molarity of the solution (M1) = ![]() M
M
(2) Calculation of Molarity of KMnO4
It is clear from the molecular equation of the reaction between KMnO4 and ferrous ammonium sulphate that 2 moles of KMnO4 oxidise 10 moles of ferrous ammonium sulphate. i.e. 10 moles of Ferrous ammonium sulphate = 2 mol KMnO4
![]()
Or M1V1 = 5 M2V2
M1 = Molarity of ferrous ammonium sulphate solution = ![]() M
M
V1 = Volume of Ferrous ammonium sulphate solution = 20 mL
M2 = Molarity of KMnO4 solution = ?
V2 = Volume of KMnO4 solution = 19.8 mL
M2 = ![]() M
M
M2 = 0.0067 M
Result: The molarity of the given KMnO4 solution is 0.0067 mol L-1
Experiment No. 3
Exercise: Find out the percentage purity of impure potassium permanganate sample. 2.0 grams of which have been dissolved in one litre solution. For this purpose you are provided with a standard solution of crystalline ferrous ammonium sulphate of molarity ![]()
Theory: Titration between potassium permanganate and ferrous ammonium sulphate is a redox titration. KMnO4 in presence of dil. H2SO4 oxidises ferrous ammonium sulphate to ferric sulphate [Fe2(SO4)3] and itself is reduced to manganese sulphate (MnSO4).
(1) Molecular equation
2KMnO4 + 3H2SO4 → K2SO4 + 2MnSO4 + 3H2O+5[0]
[2FeSO4(NH4)2SO4.6H2O + H2SO4 + [O] → Fe2(SO4)3 + 2(NH4)2SO4 + 13H20] × 5
2KMnO4+10FeSO4 (NH4)2SO4.6H2O+8H2SO4→K2SO4 + 2MnSO4 +5Fe2(SO4)3 + 10(NH4)2SO4 +68H2O
(ii) Ionic Equation :
![]()
![]()
![]()
Indicator :- KMnO4 is self indicator.
Observation table:
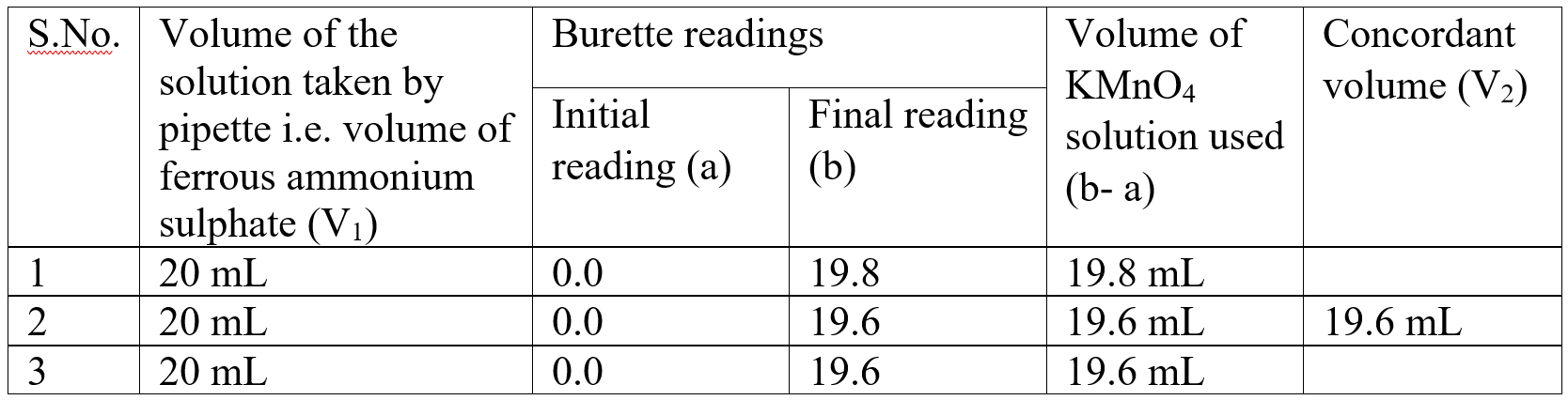
Calculations: (i) Calculation of Molarity of KMnO4
It is clear from the molecular equation of the reaction between KMnO4 and ferrous ammonium sulphate that 2 moles of KMnO4 oxidise 10 moles of ferrous ammonium sulphate. i.e. 10 moles of Ferrous ammonium sulphate = 2 mol KMnO4
![]()
Or M1V1 = 5 M2V2
M1 = Molarity of ferrous ammonium sulphate solution = ![]() M
M
V1 = Volume of Ferrous ammonium sulphate solution = 20 mL
M2 = Molarity of KMnO4 solution = ?
V2 = Volume of KMnO4 solution = 19.6 mL
M2 = ![]()
M2 = ![]()
M2 = ![]() M
M
(ii) Strength purity of KMnO4 =
Strength (gL-1) = molarity × molar mass
= ![]()
(iii) Percentage purity of KMnO4 = ![]()
= ![]()
= 53.74%
Result: The given solution of KMnO4 is 53.74% pure.

