Haloalkanes and Haloarenes
Haloalkanes: Haloalkanes are the halogen deivatives of hydrocarbons.
In haloalkanes, the halogen atom is attached to the sp3– hybridised carbon atom of an alkyl group whereas in haloarenes, the halogen atom is attached to sp2– hybridized carbon atom of an aryl group.
Classification of Haloalkanes
Monohalides (R–X): Monohalides are monohalogen derivatives of alkanes which have a general formula CnH2n+1X and are known as alkyl halides.
R–X may be of three types:
1. Primary R–CH2X
2. Secondary R2CHX
3. Tertiary R3CX
Dihalides (CnH2nX2): Dihalides are the di-halogen derivatives of alkanes and are of geminal and vicinal types.
Swarts reaction: – An alkyl chloride or bromide is heated in the presence of a metallic fluoride, such as AgF to give alkyl fluorides. This is done by heating of the alkyl chloride/bromide in the presence of the fluoride of some heavy metals
CH3Br + AgF → CH3F + AgBr
2CH3CH2Cl + Hg2F2 → 2CH3CH2F + Hg2Cl2
Swarts’ reaction is generally used to get alkyl fluorides from alkyl chlorides or alkyl bromides.
Darzen’s method (action of Thionyl chloride)-
R—OH + SOCl2 → R—Cl+ SO2↑ + HCl↑
Frinkelstein Reaction : – Iodoalkanes are easily prepared from corresponding chloroalkanes or bromoalkanes by heating with sodium iodide in acetone or methanal.
RCl + NaI ![]() RI + NaCl
RI + NaCl
The unsymmetrical alkenes follows Markovnikov’s rule during addition forming secondary or tertiary alkyl halides predominantly.
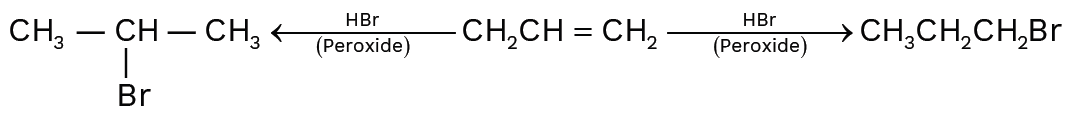
Important Points
Boiling point and density increase with increase in molecular weight.
C4H9Cl > C3H7Cl > C2H5Cl > CH3Cl
RI > RBr > RCl > RF
Order of reactivity of haloalkanes: RI > RBr > RCl > RF
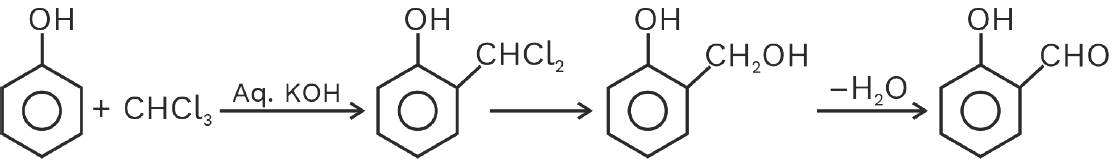
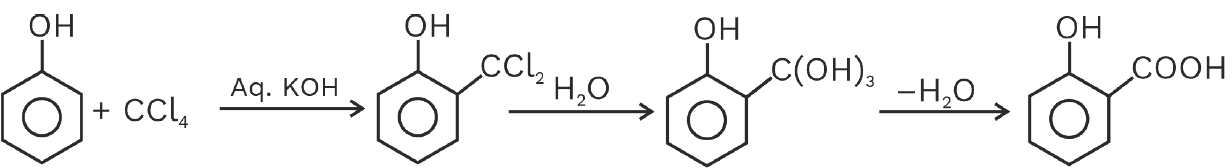
Reimer–Tiemann reaction:
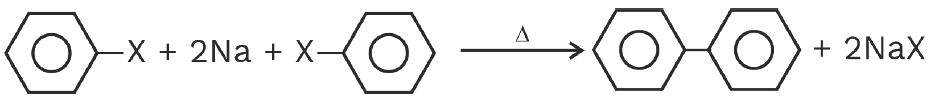
Wurtz Fittig reaction

Fittig reaction
Saytzeff’s Rule
According to Saytzeff’s rule, removal of β-Hydrogen atom takes place from β-carbon atom having more number of alkyl groups, so that a more stable alkene is formed.
Wurtz reaction: R-X + 2Na + X-R ![]() R-R + 2NaX
R-R + 2NaX
Frankland reaction
R-X + Zn + X-R ![]() RR+ ZnX2
RR+ ZnX2
Carbon tetrachloride is released into the air, it goes in the upper atmosphere and depletes the ozone layer.
What are Organo Metallics?
Ans: Organo metallic compounds are organic compounds in which metal atom is directly attached to Carbon atom.
E.g. Grignard Reagent
What is organic lead and write its uses?
Ans: Organic lead (tetraethyl lead; TEL) is used as an antiknock agent in gasoline and jet fuels.
What is difference between Geminal dihalides and vicinal dihalides
Ans: Geminal dihalides have both halide groups attached to the same carbon atom whereas vicinal dihalides have their two halide groups attached to two adjacent carbon atoms in the same compound.
Previous Year’s Question
The compound C7H8 undergoes the following reactions :
C7H8 ![]() A
A ![]() B
B ![]() C
C
The product C is
(1) m-bromotoluene
(2) o-bromotoluene
(3)3-bromo-2,4,6-trichlorotoluene
(4) p-bromotoluene
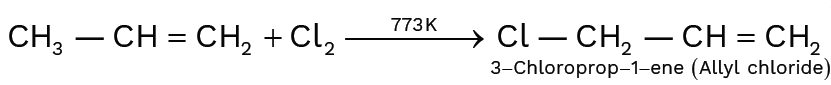
When chlorine is passed through propene at 400°C, which of the following is formed?
(1) PVC
(2) Allyl chloride
(3) Propyl chloride
(4) 1, 2-Dichloroethane
Grignard reagent is prepared by the reaction between
(1) magnesium and alkane
(2) magnesium and aromatic hydrocarbon
(3) zinc and alkyl halide
(4) magnesium and alkyl halide.
Iodoform is mainly used as an antiseptic
CHCl3 is mainly used in the production of Freon
Chloroform is kept in dark, filled, tightly closed bottle with a small amount of C2H5OH (negative catalyst) to avoid oxidation or formation of phosgene. Ethyl alcohol (C2H5OH) converts phosgene into non-poisonous diethyl carbonate.
CHCl3 + ½O2 ![]() COCl2 + HCl
COCl2 + HCl
Chloretone is used in hypnotic medicines.
Haloalkanes are less soluble in water, why?
Haloalkanes are polar molecules, neither they form H-bond with water nor can thye break the H-bonds already existing between water molecules. As a result, the solubility of haloalkanes in water in very low.
Benzene Hexachloride (B.H.C): It is commonly called as Gammexene
Out of o-and p-dibromobenzene which one has higher melting point and why?
p-Dibromobenzene has higher melting point (M.P.) than its o-isomer. It is due to symmetry of p-isomer which fits in crystal lattice better than o-isomer.
Allyl chloride is hydrolysed more readily than n-propyl chloride. Why?
Allyl chloride shows high reactivity as the carbocation formed by hydrolysis is stabilised by resonance while no such stabilisation of carbocation exists in the case of n-propyl chloride.
How can the following conversions be carried out: Propene to propan-1 -ol?
CH3 — HC= CH ![]() CH3 —CH2 —CH2 Br
CH3 —CH2 —CH2 Br ![]() CH3 —CH2 —CH2 —OH
CH3 —CH2 —CH2 —OH
Give the uses of freon 12.
Ans (i) used in aerosol propellants
(ii) refrigeration
(iii) air-conditioning.
