Download
Q.1 Correct order of –I effect is :
(1) –NR3+ > OR > F
(2) F > –NR3+ > – OR
(3) –NR3+ > F > OR
(4) OR > –NR3+ > F
Q.2 Aspirin can be prepared by the reaction of acetyl chloride with :
(1) Benzoic acid
(2) Phenol
(3) p-hydroxy benzoic acid
(4) o-hydroxy benzoic acid
Q.3 IUPAC name of 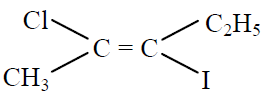 is :
is :
(1) (Z)-2-chloro-3-iodo-2-pentene
(2) (E)-2-chloro-3-iodo-2-pentene
(3) 2-iodo-3-chloro-pentene
(4) None of the above
Q.4 Which of the following does not given iodoform test :
(1) 3-pentanone
(2) 2-pentanone
(3) Ethanol
(4) Ethanal
Q.5 The product formed by the reaction of ![]() with RMgX is :
with RMgX is :
(1) RCH2–CH2OH
(2) ![]()
(3) R–O–CH2CH3
(4) ![]()
Q.6 Which of the following is not the characteristic of arenes:
(1) More stability
(2) Resonance
(3) Delocalization of π electrons
(4) Electrophilic addition
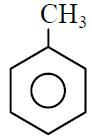
Q.7 Which of the following gives most easily electrophilic substitution reaction :
(1) 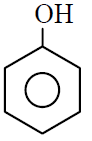
(2) 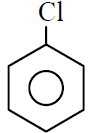
(3) 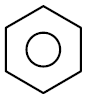
(4) 
Q.8 Which of the following does not give Claisen condensation reaction :
(1) C6H5COOC2H5
(2) C6H5CH2COOC2H5
(3) CH3COOC2H5
(4) None of the above
Q.9 Percentage of C, H & N are given as follows :
C = 40% H = 13.33% N = 46.67% The empirical formula will be :
(1) CH2N
(2) C2H4N
(3) CH4N
(4) CH3N
Q.10 Glucose +x phenyl hydrazine → osazone ‘x’ will be :
(1) 2 (2) 3 (3) 4 (4) 1
Q.11 The base found in DNA but not in RNA :
(1) Thymine
(2) Adenine
(3) Guanine
(4) Cytosine
Q.12 2-Bromo pentane reacts with ethanolic KOH gives main product :
(1) Trans-2-pentene
(2) Cis-2-pentene
(3) 1-pentene
(4) None of the above
Q.13 Which of the following does not give nucleophilic substitution with alcohol :
(1) CH3COCl
(2) Acetic anhydride
(3) Ether
(4) None
Q.14 Aniline reacts with Br2 water, NaNO2/HCl gives respectively :
(1) p-Bromo aniline, p-chloro aniline
(2) 2, 4, 6 tri bromo aniline, p-chloro aniline
(3) 2, 4, 6 tri bromo aniline, Benzene diazonium chloride
(4) p-bromo, aniline, Benzene diazonium chloride
Q.15 A complex compound which is formed by ligands nitrate and chloride. It gives two moles of AgCl precipitate with AgNO3. What will be its formulae :
(1) [Co(NH3)5NO3]Cl2
(2) [Co(NH3)5Cl]NO3Cl
(3) [Co(NH3)4Cl2]NO3
(4) [Co(NH3)4Cl NO3]Cl
Q.16 Which of the following molecule is not paramagnetic:
(1) Cu++
(2) Fe2+
(3) Cl–
(4) None of the above
Q.17 The number of antibonding electron pair in O22− is :
(1) 4 (2) 3 (3) 2 (4) 1
Q.18 When A + Water → C + B, B is reacted with D, gas C again obtained. ‘D’ gives ‘C’ with H2SO4. B gives yellow colour with Bunsen flame. C is a flammable gas then what would be A, B, C and D :
(1) K, H2, NaOH, Zn
(2) Na, NaOH, H2, Zn
(3) Li, H2, LiOH, Zn
(4) None of the above
Q.19 The concentration of ZnCl2 solution will change when it is placed in a container which is made of :
(1) Al
(2) Cu
(3) Ag
(4) None
Q.20 The cell reaction of an electrochemical cell is Cu+2(C1) + Zn → Zn+2(C2) + Cu. The change in free energy will be the function of :
(1) ln(C1 + C2)
(2) ln![]()
(3) ln C2
(4) ln C1
Q.21 A + B ![]() C + D Constant = K1
C + D Constant = K1
E + F ![]() G + H Constant = K2
G + H Constant = K2
then C + D + E + F ⇒ product. The constant of reaction will be :
(1) ![]()
(2) ![]()
(3) K1K2 (4) None of these
Q.22 Density of which of the following substance not decreases on adding in Br2 vapours :
(1) CCl4 (2) CS2 (3) Ether (4) Coke
Q.23 In which of the following molecule. The internuclear distance will be maximum :
(1) CsI
(2) CsF
(3) LiF
(4) LiI
Q.24 The fertilizer which makes the soil acidic :
(1) (NH4)2SO4
(2) Super phosphate of lime
(3) CH3COONa
(4) Ca(NO3)2
Q.25 The chiral Centre is absent in :
(1) DCH2–CH2–CH2–Cl
(2) CH3–CHD–CH2–Cl
(3) CH3–CHCl–CH2D
(4) CH3–CHOH–CH2–CH3
Q.26 Number of isomers of [Pt(NH3)4][CuCl4] complex are :
(1) 2 (2) 3 (3) 4 (4) 5
Q.27 nXm emitted one α and 2β particles, then it will become :
(1) nXm–4
(2) n–1Xm–1
(3) nZm–4
(4) None
Q.28 When X → 7N14 + 2β– then number of neutron will be in X :
(1) 3 (2) 5 (3) 7 (4) 9
Q.29 1% solution of other compound is isotonic with 5% sucrose (sugar) solution. Then molecular wt. of compound will be :
(1) 32.4 (2) 68.4 (3) 129.6 (4) 34.2
Q.30 First ionization potential of Be and B will be :
(1) 8.8 and 8.8 (2) 6.6 and 6.6 (3) 6.6 and 8.8 (4) 8.8. and 6.6
Q.31 Which of the following gives colour with the water :
(1) Cu+ (2) Cr3+ (3) Na+ (4) None
Q.32 Number of significant number will be in following numbers :
(a) 161 cm (b) 0.0161 (c) 1.61
(1) 3, 3, 3 (2) 3, 4, 3
(3) 3, 2, 3 (4) 3, 4, 4
Q.33 Maximum impurity in Pig iron will be of :
(1) Mn (2) P (3) Graphite (4) S
Q.34 Schottky defect shows :
(1) Same number of cation and decrease in anions
(2) Cations and anions are replaces from their sites
(3) Maximum number of cations and anions are same
(4) None
Q.35 Maximum oxidation state will be of :
(1) La (2) Gd (3) Eu (4) Am
Q.36 The IUPAC name of [Co(NH3)3ClBrNO2] will be :
(1) Triaminebromochloronitrocobaltate (III)
(2) Triaminebromochloronitrocobalt (III)
(3) Triaminebromonitrochlorocobalt (III)
(4) Triaminenitrochlorocobalt (III)
Q.37 By which activation energy calculate :
(1) At a constant temp.
(2) At two different temp.
(3) For reversible reaction
(4) For volatile reaction
Q.38 In the Haemoglobin (Molecular wt = 67200) iron found 0.33% (by weight). The number of iron atom will be in its one molecule :
(1) 1 (2) 2 (3) 3 (4) 4
Q.39 4NH3 + 5O2 → 6H2O + 4NO
When one mole ammonia and one mole oxygen taken :
(1) Oxygen is completely consumed
(2) Ammonia is completely consumed
(3) Both (1) and (2) are correct
(4) No one is correct
Q.40 In PO4−3 formal charge on every oxygen atom and P-O bond order is respectively :
(1) 0.75 and 1.25
(2) 0.5 and 2
(3) 1 and 1.5
(4) 0.75 and 2
Q.41 The radius of hydrogen shell is 0.53Å, then in first excited state radius of shell will be :
(1) 2.12 Å
(2) 1.06 Å
(3) 8.5 Å
(4) 4.24 Å
Q.42 Mole fraction of solute is 0.2 in solution then lowering in V.P ΔP = 10. If lowering in V.P. ΔP = 20 then mole fraction of solvent will be in solution :
(1) 0.2 (2) 0.4 (3) 0.6 (4) 0.8
Q.43 Uncertainty in position of a e– and He is similar. If uncertainty in momentum of e– is 32 × 105, then uncertainty in momentum of He will be :
(1) 32 × 105 (2) 16 × 105 (3) 8 × 105 (4) None of these
Q.44 The number of molecules of ATP produced in the lipid metabolism of a molecule of palmitic acid is :
(1) 56 (2) 36 (3) 130 (4) 86
Q.45 Identify the correct statement regarding entropy:
(1) At absolute zero of temperature, the entropy of all crystalline substances is taken to be zero
(2) At absolute zero of temperature, the entropy of a perfectly crystalline substance is +ve
(3) At absolute zero of temperature, entropy of a perfectly crystalline substance is taken to be zero
(4) At 0ºC, the entropy of a perfectly crystalline substance is taken to be zero
Q.46 The edge length of face centred unit cubic cells is 508 pm. If the radius of the cation is 110 pm, the radius of the anion is :
(1) 144 pm (2) 398 pm (3) 288 pm (4) 618 pm
Q.47 At the critical micelle concentration (CMC) the surfactant molecules :
(1) Associate
(2) Dissociate
(3) Decompose
(4) Become completely soluble
Q.48 Which one of the following pairs of substances on reaction will not evolve H2 gas ?
(1) Copper and HCl (aqueous)
(2) Iron and steam
(3) Iron and H2SO4 (aqueous)
(4) Sodium and ethyl alcohol
Q.49 The second order Bragg diffraction of X-rays with λ = 1.00 Å from a set of parallel planes in a metal occurs at an angle 60º. The distance between the scattering planes in the crystal is :
(1) 2.00 Å (2) 1.00 Å (3) 0.575 Å (4) 1.15 Å
Q.50 One mole of an ideal gas at 300 K is expanded isothermally from an initial volume of 1 litre to 10 litres. The ΔE for this process is (R = 2 cal. mol–1 K–1) :
(1) 1381.1 cal. (2) Zero (3) 163.7 cal. (4) 9 lit. atm.
