HALOALKANES AND HALOARENES:
General methods of Preparation of Alkyl halide ;
By direct halogenation of alkanes :
R-H + Cl2 ![]() R-Cl + HCl
R-Cl + HCl
By the addition of H—X on alkenes :
R-CH = CHR + HX → RCH2-CHXR
eg : CH2 = CH2 + HX → CH3-CH2X
eg : CH3 -CH = CH2 + HX →![]()
(3) By Alcohols :
(a) By the action of hydrogen halides :
R-CH2-OH ![]() RCH2-X
RCH2-X
(b) By the action of phosphorous halides :
R-OH + PCl5 → R-Cl + POCl3 + HCl
3R-OH + PCl3 → 3RCl + H3PO3
PBr3 and PI3 are less stable, thus for bromides (P + Br2) and for iodides (P + I2) mixture is used.
(c) By reaction with thionyl chloride (Darzen’s procedure) :
R-OH + SOCl2 ![]() R-Cl + SO2 + HCl
R-Cl + SO2 + HCl
(4) By halide exchange :
(Finkelstein reaction) Note : Finkelstein reaction can only be used to prepare R.I
R-Cl or R -Br + KI ![]() R-I + KCl or KBr
R-I + KCl or KBr
(Swart reaction) Note : swart’s reaction can only be used to prepare R-F.
2CH3Cl + Hg2F2 ![]() 2CH3F + Hg2Cl2
2CH3F + Hg2Cl2
Physical Properties
(a) The lower members CH3F, CH3Cl, CH3Br, C2H5Cl and C2H5F are gases at room temp.
(b) Higher B.P. than parent alkanes.
Decreasing order of B.P. is : R – I > R—Br > R—Cl > R—F
among isomeric R-X, decreasing order of B.P. is : Primary > Secondary > tertiary
(c) Decreasing order of density is: R—I > R—Br > R—Cl > R—F
(d) R-X are polar co-valent compounds but insoluble in water because they cannot form H-bonds. They dissolve in organic solvents.
(e) R-X (except R-F) burns with a green flame when interacted with Cu wire. (Beliestein test).
Uses –HALOALKANES AND HALOARENES:
(1) Dicloromethane (CH2Cl2) :
(a) Dicloromethane is widely used as a solvent as a paint remover.
(b) It is also used as a metal cleaning and finishing solvent.
(c) In humans, direct skin contact with methylene chloride causes intense burning and mild redness of skin.
(2) Trichloromethane(chloroform) (CHCl3) :
(a) Chloroform is used as general anesthetic in surgery.
(b) Chloroform is slowly oxidized by air in the presence of sunlight to an extremely poisonous gas, carbonyl chloride, also known as phosgene.
It is therefore stored in closed dark colored bottles completely filled so that air is kept out.
2CHCl3 + O2 ![]() 2COCl2 + 2HCl
2COCl2 + 2HCl
Phosgene
(3) Triiodomethane (Iodoforms) :
Iodoforms used as antiseptic due to the liberation of free iodine and not due to iodoform itself.
(4) Tetrachloromethane(CCl4) / Carbontetrachloride / Pyrin :
Used as cleaning fluid, fire extinguisher.
(5) Freons : ChloroFlouroCarbon/CFCs :
The chloro fluoro derivatives of methane and ethane are used in refrigerator
(6) DDT : Dichlorodiphenyltrichloroethane
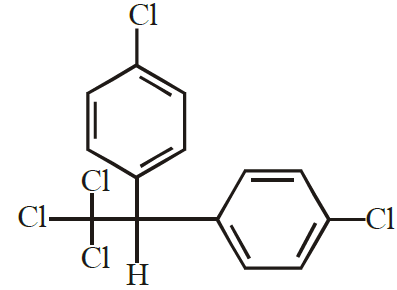
It is used as pesticides.
Toluene reacts with a halogen in the presence of iron (III) chloride giving ortho and paral halo compounds. The reaction is
(i) Electrophilic elimination reaction (ii) Electrophilic substitution reaction
(iii) Free radical addition reaction (iv) Nucleophilic substitution reaction
Ans (ii) Electrophilic substitution reaction
Chlorobenzene is formed by reaction of chlorine with benzene in the presence of AlCl3. Which of the following species attacks the benzene ring in this reaction?
(i) Cl¯ (ii) Cl+ (iii) AlCl3 (iv) [AlCl4]¯
Ans (ii) Cl+
A primary alkyl halide would prefer to undergo _____________.
(i) SN1 reaction (ii) SN2 reaction (iii) ∝-Elimination (iv) Racemisation
Ans (ii) SN2 reaction
An SN1 reaction of an enantiomerically pure chiral alkyl halide gives a product :
(a) with retention of configuration (b) with inversion of configuration
(c) with recemisation (d) with partial racemisation
Ans. (c) Racemisation
Out of the following, the one which is most reactive towards nucleophilic substitution reaction is
(A) CH2 = CH – Cl (B) C6H5 – Cl
(C) CH3CH = CH – Cl (D) CH3 – CH2 – CH2 – Cl
Ans. (D)
Haloalkanes react with KCN to form alkyl cyanides as main product while AgCN forms isocyanides as the chief product. Explain.
Ans. KCN is predominantly ionic and provides cyanide ions in solution. Although both carbon and nitrogen atoms are in a position to donate electron pairs, the attack takes place mainly through carbon atom and not through nitrogen atom since C-C bond is more stable than C-N bond. However, AgCN is mainly covalent in nature and nitrogen is free to donate electron pair forming isocyanide as the main product.
How the following conversions can be carried out ?
(i) Propene to propan-1-ol
![]()
![]()
![]()
(ii) 1-Bromopropane to 2-bromopropane
![]()
![]()
![]()
(iii) Toluene to benzyl alcohol

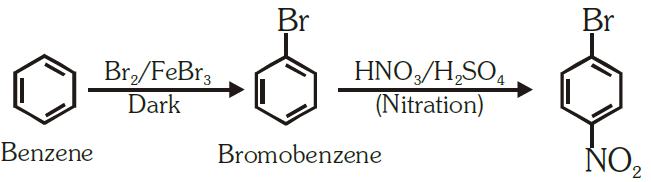
(iv) Benzene to 4-bromonitrobenzene
(v) Ethanol to propanenitrile
![]()
![]() CH3CH2CN
CH3CH2CN
(ix) Ethyl chloride to propanoic acid
![]()
![]() CH3CH2COOH
CH3CH2COOH
The treatment of alkyl chlorides with aqueous KOH leads to the formation of alcohols but in the presence of alcoholic KOH, alkenes are major products. Explain.
Ans. OH- ion is highly solvated in an aqueous solution and as a result, the basic character of OH- ion decreases. Therefore, it cannot abstract a hydrogen from the β-carbon. So
R-Cl + Aq. KOH → R-OH + KCl
On the other hand, an alcoholic solution of KOH contains alkoxide (RO-) ion, which is a strong base. Thus, it can abstract hydrogen from the β-carbon of the alkyl chloride and form an alkene by eliminating a molecule of HCl.
R-CH2-CH2-Cl + KOH(alc)→R-CH=CH2 + KCl + H2O
Give reasons:
(a) Grignard reagent should be prepared under anhydrous conditions,
(b) Alkyl halides are immiscible with water although they are polar, and
(c) Chloroform is stored in dark coloured bottles filled up to the brim.
Ans. (a) Grignard reagents in the presence of moisture, they react with H2O to give alkanes.
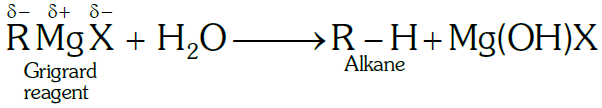
(b) The molecules of water are held together by hydrogen bonds, As the new force of attraction between water and alkyl halide molecules are weaker than the forces of attraction already existing between alkyl halide- alkyl halide molecules and water-water molecules, they cannot form H-bond with water.
(c) Chloroform is slowly oxidised by air in the presence of light to an extremely poisonous gas phosgene (carbonyl chloride). It is therefore stored in closed dark coloured bottles completely filled so that air is kept out.
2CHCl3 + O2 ![]() 2COCl2 + 2HCl
2COCl2 + 2HCl
