Qualitative Analysis of Mixture of Inorganic Salts.
Qualitative analysis — It is branch of chemistry that deals with the identification of elements or grouping of elements present in a sample. The techniques employed in this analysis vary in compulsory. Depending on the nature of the sample. It includes following principles.
Principle of Ionisation — This principle was given by anhennius. According to this principle when any electrolyte is mixed in water, it dissociates into two types of ions, one cation and alter anion. Cation is known as Basic radical and anion is known as can be detected, and the reactions are known as Ionic reactions.
Common ion effect — It is responsible for the reduction in the solubility an ionic precipitate when a soluble compound containing one of the ions of the precipitate is added to the solution in equitibion with the precipitate.
Two effect is based on la Shatelies’s principle which is applied to changes in consecration or pressure by having a content value. The effect of temperature on equilibrium, biuohes a change in equilibrium constant.
Suppose AB is a weak electrolyte
ABA+ +B-
Ionization constant K = 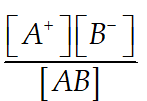
When DB which is a strong electrolyte is added to AB, it ionized as
DB D+ + B-
So, B- ion is in excess and value of K increase, so as to maintain eqm, it shift in backward direction.
If we add a slat or acid that supplies one of the ion, common to preexisting equilibrium in solution, then the equilibrium will tend to adjust by decreasing the conc. of common ion.
App. of common ion effect.
(1) HCl is added before H2S in second group Analysis
Addition of HCl (Strong electrolyte) before H2S weak electrolyte reduces the dissolution of H2S as it shifts the eqm to left side.
HCl H+ + Cl-
H2S 2H+ + S-2 ( Backward dissociation)
Thus, in solution ions are present only in that conc. so that only sulphides of Hg, Pb, Br, Cu, Cd, As, Sb and Sn get precipitate out whereas Zn, Mn, Co and Ni sulphides do not precipitate.
(2) NH4Cl is added before NH4OH in the analysis of 3 groups.
Adding NH4Cl shifts the equilibrium of NH4OH towards reactant side and than only hydroxides of Fe, Ca and Al precipitate out whereas those of Zn, Ca and Mg do not.
NH4Cl NH4+ + Cl-
NH4OH NH4+ + OH- ( dissociates in backward direction)
(3) Solubility product
Ksp = 
Solubility product for a saturated solution of sparingly so able salt can be calculated by above eqn.
Since the conc. of in solid state is taken as unity, So Ksp can be written as Ksp = 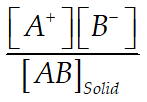
Where, Ksp is constant at constant temp. and is equal to the product of ionic conc. of ions of saturated solution of electrolyte.
(4) Ionic product
The product of conc. of ions present in any solution. On the basis of solubility product, solutions of electrolyte can be classified into three main categories.
(i) Unsaturated solution: When the value of Ionic product ( It is less than the that means KIP<Ksp
(ii) Saturated solution : When the value of ionic product is equal to that of solubility product that means KIP= Ksp
(iii) Supersaturated solution : When the value of ionic product is greater then that of solubility product KIP> Ksp
Note:- This is the condition for precipitation also.
Application of solubility product in Qualitative analysis:-
(i) Precipitation of metal chlorides of 1 groups
The group reagent for first group in qualitative analysis is dil. HCl, so that chlorides of Pb, Ag and Hg are precipitated out, they have very low value of solubility product.
S.No. Compound Ksp (at 250C)
1. PbCl2 1.6×10-5
2. AgCl 1.8×10-10
3. Hg2Cl2 3.6×10-18
Then I.P. values are greater than their Ksp values, thus precipitation takes place.
Note: (1) The difference is the values of Ksp and KIP is vary less thus is analysis of lead comes in group 1 and 2
(2) Precipitation of sulphides of metals of second and fourth group.
The group reagent of second and fourth metal ions of second and fourth group get precipitated in the form of sulphide ions.
In IInd group, H2S gas is passed in acidic medium whereas in IVth group H2S gas is passed in alkaline medium. Reason for this is that the Ksp values for second group ion are less that those of IVth group ions.
So, when H2S gas is passed in presence of HCl in IInd group, ionisation of H2S now decreases due to common ion effect and thus ionic product value of H2S also decreases which Now be able to overcome the KSP values of IInd group. ( as they are low) but cannot exceed the, KSP values of IVth group ions, so in presence of HCl, only IInd group ions can be precipitated.
Where as in the case of IVth group, they have higher KSP values and thus can be precipitated only where H2S gas is passed in the presence of NH4OH. Now the ionization of H2S gas increases, and thus their I.P. values also increases and easily exceeds the KSP values of IV th group radical and thus these ions of IV th group precipitates.
(3) Precipitation of hydroxides of 3rd group of metals ions.
The group reagent of third group radical is NH4OH it means Fe+3, Cr+3, Al+3 ions precipitates in the four of hydroxides ions. But NH4OH is added in the presence of NH4Cl , so that due to common ion effect, the ionization of NH4OH decreases solubility product of metal hydroxides of third group is less, thus even in low conc. also, ions get precipitated out and precipitation takes place. But if NH4OH is added without adding NH4Cl then the I.P. values are more and then hydroxides of Zn, Mn, Co and Mg also get precipitated out in addition to the precipitation of Fe, Cr and Al ions.
(4) Precipitation of 5th group of radicals.
The group reagent of 5th group is (NH4)2CO3 which precipitate in the form of CO3-2 ions. The order of testing radicals of 5th group is as follows Ba+2 then Sr+2 and at last Ca+2 ions.
Adding (NH4)2CO3 in the precipitate of 4th group. we get white ppt.
Dissolve this ppt in acetic acid and divide it into 3 part
I part + K2CrO4yellow ppt for Ba+2
II part + (NH4)2SO4white ppt for Sr+2
III part + (NH4)2C2O4white ppt for Ca+2
These ions are now precipitated out in the same sequence as Ba+2,Sr+2,Ca+2. Out of these BaCrO4 has lowest Ksp value, so adding K2CrO4 only BaCrO4 precipitates out, and SrCrO4 and CaCrO4 remains in solution form.
Before estimating Sr+2, Presence of Ba+2 is judged, because Ksp values of BaSO4 and SrSO4 is very less so both get precipited out.
In the same way before estimating Ca+2, Ba+2 and Sr+2 ion can be judged because, BaC2O4,SrC2O4 and CaC2O4 have low Ksp values and thus all these gives white ppt.
So, for the detection of Ca+2 ions Ba+2 precipitate as BaCrO4 and Sr+2 precipitate as SrSO4 .
Radicals: When any change is present on any atoms or group of atoms, is called radical.
There are 2 types of radicals:
(1) Acid radicals or Anions: – When negative charge is present on any atoms or group of atom are called acidic radicals. They are divided into following 3 group on the basis of Response with dil. H2SO4 or conc. H2SO4
(a) Weak acidic group: – In this group, when dil. H2SO4 is added, it gives some specific gas which may how specific colour also. This group includes CO3-2 (Carbonate ion), SO3-2 (Sulphite ion), S-2
(Sulphide ion), NO2- (Nitrite ion) and CH3COO- (acetate ion).
(b) Strong acidic group:– Radicals of this group, gives specific colour and odour with conc. H2SO4 . It includes Cl- (Chloried ion), Br- (Bromide ion), I- (Iodide ion), NO3- (Nitrate ion) and C2O4- (Oxalate ion).
(c) General acidic group:– Radicals of this group, are not decomposed by dil. H2SO4 or conc. H2SO4 . They are SO4-2 (Sulphate ion) and PO4-3 (Phosphate ion).
(B) Basic radical:- Atom or group of atoms which have positive charge on it are called basic radicals. They are classified into 7 groups (from Zero to Sixth)
Acidic Radicals
|
Group |
Group Reagent |
conclusion |
Name of Radicals |
|
1 |
dil. H2SO4 |
Evolution of Gases and characteristic smell |
CO3-2 , SO3-2 , S-2 , NO2-2 , CH3COO- |
|
2 |
Conc. H2SO4 |
Evolution of Gases and characteristic smell and colour |
Cl- , Br- , I- , NO3-2 , C2O4-2 |
|
3 |
Dil. HCl+BaCl2 |
White ppt. |
SO4-2 |
|
4 |
Conc. HNO3+ Ammonium Molybatate |
Yellow ppt. |
PO4-3 |
Preliminary test-
Group I (Weak Acidic Group)
Radicals – CO3-2 (Carbonate ion), SO3-2 (Sulphite ion), S-2 (Sulphide ion), NO2- (Nitrite ion) and CH3COO- (acetate ion).
Reagent- Dil. H2SO4 or Dil HCl
Take small amount of salt in a dry test tube and add 2 ml of dil. H2SO4 or dil. HCl. Heat the contents if necessary (do not boil). Now, observe the evolved gas.
|
Sr. No. |
Observations |
Anion suspected |
Confirmatory test |
|
1 |
Brisk effervecence with the evolution of a colourless gas (CO2) |
Carbonate CO3-2 |
Pass the evolved gas into lime water. It turns milky due to formation of CaCO3. and becomes colourless when gas is passed in excess due formation of Ca(HCO3)2 |
|
2 |
Colourless gas (SO2) wit suffocating smell of burning sulphur. |
Sulphite SO3-2 |
Put a filter paper moisture with acidified K2Cr2O7 solution at the mouth of the test tube, it turns green due to formation of Cr2(SO4)3. |
|
3 |
Colourless gas with the smell of rotten eggs. |
Sulphide S2- |
(i) Put a filter paper soaked with lead acetate solution at the mouth of test tube. it turns black due to formation of PbS. (ii) The evolved gas turns of filter paper moisture with sodium nitroprusside, Na4[Fe(CN)5NO]. (iii) The evolved gas turns of filter paper moisture with Cadmium acetate solution, yellow due to formation of CdS. |
|
4 |
Smell of vinegar (CH3COOH) |
Acetate CH3COO- |
(i) Rub a small amount of the mixture with oxalic acid and a few drops of water on your palm. A smell of vinegar. (ii) Shake a small amount of the mixture in water and filter. To the filtrate add ferric chloride solution. A blood red colour is obtained due to formation of (CH3COO)3Fe. |
|
5 |
Reddish brown vapours (NO2 gas) with pungent smell. |
Nitrite, NO2- |
The evolved gas turns a filter paper soaked with KI and starch solution (acidified) to blue-black due to liberation of iodine which forms blue iodostarch complex. |
Group II (Strong Acidic Group)
Radicals: Cl-,Br-,I-,NO3-,C2O4-2
Reagent- Conc. H2SO4
Take small amount of salt in a dry test tube and add 2 ml of Conc. H2SO4 . Heat the contents if necessary (do not boil). Now, observe the evolved gas.
|
Sr. No. |
Observations |
Anion suspected |
Confirmatory test |
|
1 |
Colourless pungent gas (HCl) comes out. |
Chloride Cl- |
(i) Bring a glass rod dipped in silver nitrate solution on the mouth of test. White curd ppt. on the rod. (ii) A glass of NH4OH is brought to the mouth of test tube white fumes of NH4Cl are produced. (iii) CHROMYL CHLORIDE TEST: Mix the solid substance three times its weight K2Cr2O7 in a dry test tube. Add Conc. H2SO4 . Gently warm the mixture and pass the deep red vapours chromyl chloride in NaOH solution contained in a test tube. Acid the yellow solution with acetic acid and add lead acetate solution. A yellow precipitate confirms the presence of chloride. |
|
2. |
Pungent colourless or reddish brown vapours turning the liquid red (Br2) |
Bromide Br- |
(i) Mix. + Conc. H2SO4 +MnO2. Red brown vapours of bromine are evolved. (ii) Solution of mixture or sodium carbonate extract + HNO3 + AgNO3. Pale yellow ppt. partly soluble in NH4OH. (iii) To the sodium carbonate extract add dil. H2SO4. Cool. add chloroform or carbon tetrachloride and then excess of chlorine water with shaking. The chloroform layer becomes orange red or red brown. |
|
3. |
Dark violet fumes are evolved which condense on the upper part of the test tube |
Iodide I- |
(i) Bring a starch paper at the mouth of test tube. It turns deep blue. (ii) Solution of mixture or sodium carbonate extract + HNO3 + AgNO3. Pale yellow ppt. insoluble in NH4OH. (iii) To the sodium carbonate extract add dil. H2SO4. Cool. add chloroform or carbon tetrachloride and then excess of chlorine water with shaking. The chloroform layer becomes turns violent or purple. |
|
4. |
Light brown fumes having pungent smell evolved . The vapours increase on addition of Cu turnings in solution. |
Nitrate NO3- |
(i) Ring Test: To 1 ml aqueous solution of sodium carbonate extract (acidified with dil H2SO4). Add 2 ml Conc. H2SO4) cool and saturated ferrous sulphate solution slowly from the side of test, Now allows to stand for a minute. Brown ring forms a junction of two layers due to formation of [FeSO4.NO] |
|
5. |
Evolution of odourless, colourless vapours which burn with blue flame and turn lime water milky. |
Oxalate, C2O42- |
(i) Dissolve a small amount of the mixture in dil. HCl or H2SO4. Add a few drop of KMnO4 solution. Decolourisation occurs (pink colour disappear). (ii) Take 2 ml Sodium carbonate extract and add excess of acetic acid to it. Add CaCl2 solution in excess and allow to stand for some time. Filter the white ppt. and extract it with hot dil. H2SO4. Now add to it a few drops of dilute KMnO4 solution. The pink colour of KMnO4 disappears. |
(c) General acidic group:–
Radicals of this group, are not decomposed by dil. H2SO4 or conc. H2SO4 . They are SO4-2 (Sulphate ion) and PO4-3 (Phosphate ion).
|
Sr. No. |
Observations |
Anion suspected |
Confirmatory test |
|
1. |
The mixture is boiled with conc. HNO3. Now add ammonium molybdate [(NH4)2MoO4] solution and coll. A canary yellow ppt. appears. |
Phosphate PO43- |
Precipitate is soluble in ammonium hydroxide and alkali hydroxides. |
|
2. |
In the solution of the mixture in dil. HNO3 add BaCl2 solution. A curdy white ppt. is formed. |
Sulphate, SO42- |
(i) Precipitate is insoluble in all concentrated acids. (ii) In the solution of the mixture add lead acetate solution. White ppt of PbSO4 is formed. (iii) Take a portion of the sodium carbonate extract and acidify it with dil. HCl. Now add BaCl2 solution in it. A white ppt. is formed which is insoluble in all the concentrated acids. |
Sodium Carbonate Extract: Sodium carbonate extract in aqueous medium is used in the analysis of acid radicals. Sodium salt of most of the acid radicals insoluble in water. Sodium carbonate extract or soda extract is the filtrate obtained after boiling the salt or mixture with excess of sodium carbonate in distilled water.
Method of preparation:- For preparing soda extract mixture (salt) is mixed with about four times of pure sodium carbonate (free from chloride and sulphate ions), 20-25 ml distilled water is added and boiled for about 20 minutes and filtered.
Chemistry involved in Sodium Carbonate Extract: On boiling the salt with Na2CO3. solution, double decomposition takes place; cations are converted to their insoluble carbonate residue and anions form soluble salts with sodium. Filtrate which is called as soda extract contain sodium salt of anions of excess of Na2CO3.
MgSO4 + Na2CO3 MgCO3 + Na2SO4
Insoluble Soluble
CdCl2 + Na2CO3 CdCO3 + 2NaCl
Insoluble Soluble
BaCl2 + Na2CO3 BaCO3 + 2NaCl
Insoluble Soluble
Al2(SO4)3 + 3Na2CO3 +3H2O 2Al(OH)3 + Na2SO4 + 3CO2
Insoluble Soluble
Excess sodium carbonate present in the soda extract should be neutralized with appropriate acid. It should be kept in mind that neutralization should be done with the acid which does not contain the anion under test. For example, it would be futile to neutralize the extract with HCl if chloride is to be tested.
Basic Radicals:
Identification of the Cataion on the basis of Colour of salt.
|
Cations |
compounds |
conditions |
colour |
|
Ag2+ |
AgCl ( white). AgBr and AgI ( Yellow) AgNO3 ( white). AgF ( white). |
They turn black on exposure to sunlight, on reduction to Ag. | |
|
Hg22+, Hg2+ |
Hg2O, HgS ( Black) Hg2Cl2, HgCl2, HgSO4 (White).HgO, HgI2(Red) |
HgS turns red on heating called vermilion | |
|
Cu2+ |
Copper salts are blue green |
Blue green | |
|
Fe3+ |
Reddish brown salts. | ||
|
Co2+ |
CoO (Green). CoCl2(Pink) Co2O3 (Brown) CoCl2 (Anhydrous)- Blue. Co(NO3)2 , CoSO4 (Blue) | ||
|
Zn2+ |
ZnCO3 (White)Zno (White) |
yellow on heating | |
|
Ni2+ |
Blue-green salt, NiO- Green NiSO4.7H2O (Blue) | ||
|
Ni3+, Ni2+ |
Black salt Ni2O3, NiO2.2H2O (Black), NiS (Black). NiSO4.7H2O (Blue) | ||
|
Mn2+ |
Light pink, black or colourless compound MnCl2-Pink MnO2-Black KMnO4-Violet MnO-Green Mn2O3-Brown Mn(OH)2-White |
No effect of heat on colours |
ANALYSIS OF BASIC RADICALS
Group I
Radicals : Ag2+, Pb2+, Hg22+(ous)
Group Reagent: Dil. HCl
Precipitate: AgCl, PbCl2, Hg2Cl2 (White preciptate)
Analytical Procedure: Take cold dilute original solution and add dropwise HCl is white preciptatte is form HCl so as to complete the precipitation of first group basis radicals. If precipitate is not formed then first group of PbCl2 or Hg2Cl2) is used for first group analysis.
If salt is insoluble in dil. HCl then try dissolve it in conc. HCl (first in cold condition and then boil it). Add some water in the solution. reject the insoluble portion by filtering. Filtrate is used for basis radicals analysis.
|
Sr. No. |
Slovent |
Salts whiel dessolve |
|
1. |
Cold water |
(i). All NH4+, Na+, K+ salts. (ii). All nitrites, nitrates and acetates. (iii). Most of sulphates except those of Pb, Ba, Ca, Sr (iv) All chlorides except that of lead. |
|
2. |
Hot water |
Lead chloride, lead nitrate and all above salts. |
|
3. |
Dil. HCl |
All carbonates which do not dissolve in water. i.e. Ca, Ba, Sr, Mg, Zn, Al,Ni,Cu, Mn, Fe etc, but not of Pb |
Classification of Basic or Matalic Radicals
|
Group |
Cation |
Group reagint |
precipite |
Flam test |
|
0 |
NH4+, Na+, K+ |
K2[HgI4] (Nessler’s reagent) |
Na- Golden yellow K- Violet Li-Crimson red | |
|
1. |
Ag+, Hg22+, Pb2+ |
dil. HCl |
AgCl, Hg2Cl2, PbCl2 | |
|
2. |
Hg2+, Cu2+,Pb2+, Bi2+, Cd2+, Sn2+, Sb2+, As3+, Sn2+ |
Dil HCl + H2S gas or Na2S |
HgS, Cus, PbS, Bi2S3, CdS, SnS, Sb2S3, As2S3, SnS, | |
|
3. |
Fe3+, Al3+, Cr3+ |
NH4Cl + NH4OH |
Fe(OH)3, Al(OH)3, Cr(OH)3 | |
|
4. |
Zn2+, Co2+, Mn2+, Ni2+ |
H2S in presence of NH4Cl and NH4OH |
ZnS, CoS, MnS, NiS | |
|
5. |
Ba2+, Sr2+, Ca2+ |
(NH4)2CO3 in presence of NH4Cl and NH4OH |
BaCO3, SrCO3, CaCO3 |
Ba- Apple green Sr- Brick red Ca- Crimson red |
|
6. |
Mg2+ |
Na2HPO4 in presence of NH4Cl and NH4OH |
Mg(NH4)PO4 |
Mg- Blue colour with K4[Fe(CN)6] |
Classification of cations into analytical groups depends on solubility of their chlorides, sulphides, hydroxides, carbonates in water and variouos other reagenst.
Classification of Basis Radical
This test is only positive in absence of Zn2+ and Fe3+.
ANALYSIS OF BASIC RADICALS
Group 1
Radicals: Ag+, Pb2+, Hg22+(ous)
Group reagent: Dil HCl
Precipitate: AgCl, PbCl2, Hg2Cl2 ( White precipitate)
Analytical procedure: Take cold dilute original solution and add dropwise dil. HCl, if white precipitate is fromed add more dil HCl so as to complete the precipitation of first group basic radicals. If precipitate is not formed then first group is absent.
Now, filter the precipitate: keep the filtrate for IInd, IIIrd and higher group analysis. The precipitate (AgCl or PbCl2 or Hg2Cl2) is used for first group analysis.
|
wash the precipitate with cold water and then boil with water and filter it whle filter boiling. | |
|
Filtrate may contain Pb2+ ( Divide it in four parts)(i). First part : White crystalline ppt of PbCl2 reappears on cooling.(ii) Second part: Add K2CrO4 to get yellow ppt. of PbCrO4 insoluble in dil. CH3COOH but soluble in aq. NaOH.(iii) Third part: Adding KI gives yellow ppt. of PbI2 insoluble in cold water.(iv) Fourth part: Add dil H2SO4 and ethyl alchol, it gives white ppt of PbSO4 soluble in saturated ammonium acetate. (Pb2+ confirmed) |
Residu may contain AgCl and Hg2Cl2. boil it with water and three again filter. Treat the precipitate with NH4OH and filter. |
|
Filtrate: AgCl ppt dissolve forming complex [Ag(NH4)2]Cl. Divide the solution into four part(i) In first, dil. HNO3 forms white ppt which confirms Ag+.(ii) In second part add KI solution which gives yellow ppt fo AgI ( Ag+ Confirmed)(iii) In third part add excess of acetic acid followed by K2CrO4 gives brick red ppt which confirms Ag+(iv) On Passing H2S gas to foouth part we get black ppt which is insoluble in NH4OH but soluble in dil HNO3 ( Ag+ Confirmed) |
Black resude may be of [Hg(NH2)Cl+ HgI] Dissolve the precipitate in aqua-regia and divid in to two parts.(i) On adding SnCl2 to first part we get white ppt. which turns black on standing. (Hg+ Comformed)(ii) Add Copper turning to second part, layer of grey residue deposits.(Hg+ Comformed) |
Precautions in First Group Analysis
1. Do not add conc. HCl beacuse chlorides of lead and silver are soluble in conc. HCl.
2. Excess of HCl should be avoided beacuse PbCl2 dissolves due to complex formation.
HCl + PbCl2H[PbCl2]
2HCl + PbCl2H2[PbCl2]
3. Some soluble lead ion goes to the filtrate of first group hence precipitated as sulphate in second group when H2S gas is passed.
Note:
1. Precipitate of PbCl2 is soluble in how water and unsoluble in cold water. However, precipitate of AgCl and Hg2Cl2 are insoluble both in hot and cold water.
2. If original solution si prepared in dil HCl then first group is absent.
3. Solubility products of frist group halides (AgCl, PbCl2, Hg2Cl2) are smaller than higher group halides. Hence, dil HCl precipitates first group halides. We know that for precipitation ionic product should exceed solubility product.
KIP > KSP ( Condition for precipitation)
Compound Solubility product
PbCl2 1.6×10-5
AgCl 1.6×10-10
Hg2Cl2 1.1×10-16
Group IIA
Radicals: Hg2+, Pb2+, Bi2+, Cu2+, Cd2+
Analytical Procedure: Take the precipitate of insoluble in hot yellow ammonium sulphide) in a test tube and add 5 ml of dil. HNO3. Boil for few minutes. If insoluble, filter it.
|
Insoluble (black) HgS. Dissolve it in aqua-regia and divide into three parts. Part I. Add SnCl2 solution. A white ppt. which changes into greyish coloured ppt. Part II. Add Cu turnings. White shining deposit on copper turning. Part III. Add potassium iodide solution, a bright which dissolves in excess of potassium iodide solution. [Hg2+ (ic) is confirmed] |
Filtrate: It may have Pb2+, Cu2+, Bi2+ or Cd2+. Divide the solution into two parts. First part: Add dil. H2SO4 and ethyl alcohol. The appearance of white precipitate confirms the presence of lead. In case no precipitate, use second part of filtrate. Dissolve white precipitate of PbSO4 in saturated ammonium acetate. Add dil. acetic acid in the solution and then K2CrO4; yellow ppt of lead chromate confirms Pb2+. Second part of filtrate – Excess of NH4OH solution. White precipitate (then filter) or blue coloured solution or colourless solution. | |
|
White precipitate Dissolve the ppt. in dilute HCl and divide into three parts. Part I. Add sodium stannite solution; Black ppt(Bi2+ is Confirmed) Part II. Dilute with water.white ppt. (Bi2+ is Confirmed) Part III. Add a little 10% thiourea solution. Yellow colouration.(Bi2+ is Confirmed) |
Black ppt. Acidify the solution with acetic acid and add K4Fe(CN)6 solution. Chocolate coloured ppt.(Cu2+ is Confirmed) On adding iron turnings to the solution, red residue deposits on the surface.(Cu2+ is Confirmed) Before adding iron turinigs acidify the solution by H2SO4(Cu2+ is Confirmed) |
Colourless solution(Copper is absent)Pass H2S gas through solution. Apperance of yellow ppt(Cd2+ present)On adding KCN white ppt. is formed which dissolves in excess of KCN. When H2S gas is passed through this solution; yellow ppt. of CdS reappears.(Cd2+ present) |
Group II B
Radicals : Filtrate of II A group may contain following thio-salts.
(NH4)3AsS4, (NH4)2SbS4, (NH4)2SnS4 ,
Analytical Procedure: Add dil HCl first to make the medium acidic, warm, filter and wash, reject the filtrate. The residue over the filter paper contains As2 S4, Sb2 S4, Sn2 S4 . transfer the precipitate in beaker (or test tube); boil it with conc. HCl for about 5 minutes, filter and wash.
|
Residue(As2S3 or As2S5) |
Filtrate ( contains SbCl3 and SnCl4) | |
|
(i) Dissolve the yellow ppt. in conc. HNO3 and add ammonium molybdate and heat. Canary yellow ppt. (As3+ Present) (ii) Dissolve yellow ppt. in saturated solution of ammonium carbonate. Add dil. HCl in solution and pass H2S gas. Yellow ppt. (As3+ Present) (iii) Dissolve the yellow precipitate in conc. HNO3; Add excess of ammonia solution and then magnesia mixture.(As3+ Present) |
(i) In one part of solution, add NH4OH to neutralise and pass H2S gas. Orange red ppt.(Sb3+ present) (ii) In second part of solution, Add NH4OH to neutralise and add excess of water. white ppt.(Sb3+ present) (iii) In the third part, add iron turnings. Black ppt.(Sb3+ present) |
Divide rest of this solution in three parts. (i) Add mercuric chloride to the first part of filtrate. White ppt. turning black.(Sn4+ is Confirmed) (ii) To the second part of filtrate add little NaOH. White ppt. soluble in excess of NaOH.(Sn4+ is Confirmed) (iii) Add few drops of FeCl3 and K3[Fe(CN)6] which gives brown coloured solution. Add third part of solution to above brown solution. prussian blue solour. (Sn4+ is Confirmed) |
Note: When K3[Fe(CN)6] and FeCl3 are added in acidic solution of SnCl2 we get prussian blue colour.
K3[Fe(CN)6] + FeCl3 + 3SnCl2 3 SnCl4 + 2Fe4[Fe(CN)6]3 + 18 KCl
Group III
Rediacls: Fe3+,Al3+,Cr3+
Analytical Procedure: Boil off H2S from the filtreate of III group or take original solution if first and second group radicals are absent. Add few deops of conc. HNO3 and boil the solution. Add 10 gm solid ammonium chloride and ammonium hydroxide slowly till the solution smells of ammonia. If the precipitate appears, filter. the filtrate should be kept for subsequent groups.
|
Reddish brown ppt. Fe(OH)5 |
Green ppt.Cr(OH)5 |
White ppt.Al(OH)5 |
|
Dissolve the ppt in dil. HCl and divide in two part. Part I – K4[Fe(CN)6] solution. Prussian blue solution or ppt. of ferric ferrocyanide.Part II- KCNS solution. Red colouration of ferric thiocyanate.(Fe3+ Present) |
Green ppt. + NaOH + bromine water. Yellow solution. Acidify the yellow solution with acetic acid add lead acetate solution. Yellow ppt of PbCrO4 Acidify the yellow solution with dil. H2SO4 and add small amount of ether sometimes the ether layer turns blue due to dissociation of unstable perchromic acid. (Cr3+ Present) |
Dissolves the ppt in dil. HCl add NaOH solution Which ppt appears which then dissolves in excess of NaOH due to formation fo NaAlO2. Treat this solution with solid ammonium chloride Gelatinous white ppt. Dissolve the ppt. in dil. HCl and moisten the piece of filter paper with the solution and a few drops of Co(NO3)2. On burning the filter papter over bunsen flame we get blue coloured ash. (see the chemistry of cobalt nitrate test in detail in the chemistry of III group)(Al3+ Present) |
Distincton test of Fe2+ and Fe3+
|
Reagent |
Ferrous (Fe2+) |
Ferric ( Fe3+) |
|
KCNS |
No colour |
Blood red colour discharged by Hg2Cl2 |
|
K3[Fe(CN)6] |
Ferroous ferric cynadid (Tumbll’s blue ppt) |
No ppt but brown colour of ferric ferrncynide |
|
K4[Fe(CN)6] |
Ferrous ferrncyanide white pale blue ppt |
Ferric ferrocynaide(Prussian blue ppt) |
Note:
1. H2S must be completely removed otherwise the sulphides of the IV group will be precipited here.
2. A very small amoutn of HNO3 should be used otherwise manganese from divalent state (Mn2+) is oxidised to trivalent stste (Mn3+) which may get precipatedas Mn(OH)3
3. Precipitation should be done in hot condition and large excess of NH4OH should not be used. Ammonium cholride should always be added before the addition of ammonium hydroxide.
4. (NH4)2SO4 cannot be used at the place of NH4Cl because barium sulphate if present.
5. Excess of NH4Cl should be added otherwise, manganses of IV group will be precipitated as MnO2.H2O
6. NH4NO3 cannot be used in place of NH4Cl because NO3- ion is an oxidiser and will oxidise Mn2+ to Mn3+ and will bring the precipitation of Mn(OH)3 in this group.
7. It is necessary to oxidise Fe2+ to Fe3+ by conc. HNO3; because in presence of NH4Cl, ferrous hydroxide will not be precipitated fully. However ferric ions will be fully precipitated. It is so because KSP of Fe(OH)3 is less than KSP of Fe(OH)2
KSP Fe(OH)2 = 4.8×10-16; KSP Fe(OH)3 = 3.8×10-38
8. Excess of NH4Cl will bring ouot the formation of complex salts like Fe2(ZnO2)3; . thus, hydroxides of third group will not be preciptated.
9. Excess of NH4Cl creates problem in complete precipitation of Ba2+, Sr2+and Ca2+ in Vth group because excess of NH4+ ions reduce the concentration of CO32- ions.
+NH4+ +NH3
NH4+ions shift the above equilibrium, thereby reducing the concentration of (Le Chatelier’s Principle)
10. Before adding NH4OH as reagent in third group, the solution should be kept warm to avoid pink coloured complex [Cr(NH3)6](OH)3.
11. Sometimes white precipitate isformed even when Al3+ is absent. It is due to presence of sodium silicate impurity.
Na2SiO3 +NH4Cl SiO2 + H2O+2NaCl + 2NH3
Sodium silicate White ppt.
11. if NH4OH is added directly in third grooup analysis, hydroxides of IV and V group may also be precipitated. To avoid this problem NH4Cl is added prior to NH4OH which lowers the OH- ions, only IIIrd group hydroxides are precipitated whereas IV and V group radicals remain in the solution.
Moreover, NH4Cl avoids the precipitation of double hydroxides.
Fe(OH)3,Ni(OH)2
Group IV
Rediacls: Ni2+,Co2+,Mn2+,Zn2+
Group reagent: H2S gas in presence of NH4Cl and NH4OH
Analytical Procedure: Take the filtrate of third grooup, concentrate it by heating. Add amonium hydroxide and again hear the solution. Pass H2S gas. If the precipitate appears, Pass H2S gas for sufficient time and then filter. Keep the filtrate for subsequent groups.
|
Black precipitate, NiS or CoS |
Buff coloured precipitate, MnS |
White precipitate, ZnS |
|
Wash the Ppt, with how water and dissolve it in aqua-regia and evaporate to dryness.Extract the residue with water of tilute HCl. Part I + dimethyl glyoxime + NH4OH. Scarlet red ppt.(Ni2+ Confirmed) Part II + NH4Cl(solid) + Acetone. Blue layer (Co2+ Confirmed) Part III. In third part add solid NaHCO3 and Br2 water. Green colour on heating(Co2+ Confirmed) Part IV: Add excess of acetic acid and then add NaNO2. Yellow crystalline precipitate of sod. Cobaltinitrite Co2+ Confirmed) |
Dissolve in dil. HCl. Add NaOH to the clear solution and then Br2 water. Boil and filter. The ppt. is treated with conc. HNO3 and PbO2 or Pb3O4 (red lead). The contents are heated. Keep the test tube for some time. Purple colour solution.(Mn2+ present) To the second part of precipitate add the mixture (Na2CO3 + KNO3) and heat it in procelain dish . Green mass is obtained which gives pink solution in water(Mn2+ present) |
Dissolves the ppt in dil. HCl add NaOH drop by drop. The precipitate formed dissolves in NaOH. Pass H2S gas through first part of this solution. Appearance of white ppt.(Zn2+ Present) To the second part of solution add K4[Fe(CN)6] solution in excess. White precipitate.(Zn2+ Present) |
Note:
1.Do not pass H2S for more than 1 minute because excess of H2S will bring out the colloidal precipitation of NiS whose filtration is difficult.
2. If the colour of precipitate is yellow it will be of CdS.
3. If salt is white and colour of precipitate in IVth group is black; it will be of PbS.
4. Filter the precipitate of ZnS/MnS while hot otherwise precipitate particle may pass out through the pores of filter paper.
NH4OH converts ammonium bicarbonate to ammonium carbonate.
NH4HCO3 + NH4OH (NH4)2CO3+ H2O
5. Before precipitation of V th group radicals warming of solution at about 500C is necessary to decompose bicarbonates into carbonates.
6. Do not dissolve the precipitate of Vth group in excess of CH3COOH. Excess of actic acid slows down the precipitation of BaCrO4.
7. By the addition of (NH4)SO4 both CaSO4 and SrSO4are precipitated but excess of (NH4)SO4 from soluble complex with CaSO4 where SrSO4 is precipitated.
8. Sequence of precipitation of Vth group radicals is
Ba2+ Sr2+ Ca2+
9. It is so because like SrSO4 , BaSO4 is also soluble in acetic acid, like CaC2O4 ; BaC2O4 ; and SrC2O4 are also soluble in acitic acid.
(a) If Ba2+ is present; Test of Ca2+ and Sr2+ is not necessary.
(b) If Sr2+ is present; Test of Ca2+ is not necessary.
Group V
Redicals: Ba2+,Sr2+,Ca2+
Group reagent: (NH4)2CO3 in presence of NH4Cl and NH4OH
Analytical Procedure: Take the filtrate of fourth grooup. Boil off H2S gas and concentrate to about 1/2 of its volume. Add a little solid NH4Cl and NH4OH followed by (NH4)CO3 solution.Appearance of white ppt. shows the presence of V group. Filter and keep th filtrate for VI group. The white ppt may be either of BaSO4 ,SrSO4 or CaSO4 . Dissolve the precipitate in minimum quanitity of acetic acid. Divide the solution in three parts. In the solution first of all Ba2+ ,and then Sr2+ and lastly Ca2+ is tested bacause their solubility product lie in same sequence.
|
Ist part biol+ K2CrO4 solution dropwise . Yellow precipitate Dissolve yellow precipitate in minimum volume of conc. HCl and do the flame test for barium. Apple green flame.(Ba2+ Confirmed) |
If Ba2+ is absent, use IInd part. Add (NH4)2SO4 solution . White precipitate Dissolve white precipitate in minimum volume of conc. HCl and do the flame test for stronitiium. Crimson red flame.(Sr2+ Confirmed) |
If Ba2+ and Sr2+ both are absent, use the IIIrd part Add (NH4)2C2O4 solution dropwise .white precipitate Dissolve white precipitate in minimum volume of conc. HCl and do the flame test for barium. Brick red flame.(Ca2+ Confirmed) |
Note:
1. Filtrate of IV group should be acidified with acetic acid and boiled to evaporate H2S gas. Otherwise (NH4)2SO4 by atmospheric oxygen. (NH4)2SO4 thus formed will therefore precipitate out insoluble BaSO4 ,SrSO4 and CaSO4 . In presence of acetic acid, H2S very fastly.
2. (NH4)2CO3 in presence of NH4Cl is used as reagent. Excess of NH4Cl decreases ionisation of (NH4)2CO3 and hence lowers the concentration of CO3-2 ions because both salts have common NH4+ ion.
NH4Cl +Cl-
(NH4)2CO3 2+
3. If NH4Cl is not added before addition of (NH4)2CO3 then there will be precipitation of basic magnesium carbonate [4MgCO3.Mg(OH)2.5H2 O ].NH4Cl reduces the CO3-2 ion concentration by common ion effect. thus, ionic product of magnesium basic carbonte does not exceed solubility product and precipitation does not take place.
4. Presence of NH4OH is also essential along with NH4Cl before of (NH4)2CO3 becaues NH4HCO3 present with (NH4)2CO3form soluble bicarbonates, viz. Ca(HCO3)2 ; Ba(HCO3)2 ; Sr(HCO3)2 .
5. Solubility product of IV group sulphides are:
Compound KSP
MnS 2.3×10-38
CoS 4×10-22
ZnS 2.5×10-22
NiS 3×10-21
6. In the analysis of cobalt do not add KSCN because Fe3+ of IIIrd group may precipitate as blood red coloured Fe(SCN)3
FeCl3 + 2KSCN Fe(SCN)3 + 3KCl
Group VI
Radicals: Mg2+, Na+, K+
Group reagent: Na2HPO4 in presence of ammonium chloride and ammonium hydroxide.
Analytical procedure : Add 3-4 ml ammonium oxalate solution to the Vth group filtrate, boil and filter. Reject precipitate if any. To the filtrate add Na2HPO4 solution. Heat slowly for few minuts. Scratch the side of test tube with a glass is A white crystalline precipitate of Mg(NH4)PO4 will be formed slowly.
Note:
1. Magnesium ammonium phosphate Mg(NH4)PO4 will be precipitated in presence of NH4Cl and NH4(OH)2
2. Before testing Mg2+ , one should be sure that the radicals from first to fifth group are absent. The reagent Na2HPO4 may form insoluble precipitate with the radicals of first to fifth group.
3. Precipitation of Mg(NH4)PO4 takes place very slowly. On scratching the wall of test tube with glass rod, we speed up the process precipitation.
Specific test of Mg2+ (Blue Lake Test)
The magneson reagent, i.e. p-nitrobenzen-azo-resorcinol or p-nitrobenzene-azo-naphthol is add to Vth group filter after making alkaline by NaOH solution. It is observed upon Mg(OH)2 forming sky blue precipitate or lake.
|
S.N. |
Observation / Experiment |
Inference |
Conclusion |
|
1. |
To a part of the original solution, add potassium permanganate solution and rub the sides of the test tube with glass rod. |
White milkyness |
Na+ conformed |
|
2. |
To a part of original solution add NaOH solution to make it alkaline and then add freshly prepared sodium cobalt nitrite solution. |
Yellow precipitate |
K+ Conformed |
Note
Na+ and K+ are not included in the syllabus of CBSE board.
Zero Group
|
S.N. |
Observation / Experiment |
Inference |
Conclusion |
|
1. |
On adding caustic soda solution to the mixture |
Smell of NH3 is observer |
NH4+ may be |
|
2. |
On placing glass rod mist with HCl after adding caustic soda to the mixture |
White fumes of NH4Cl if formed |
NH4+ May be |
|
3. |
On placing red litmus on the mouth of test tube after adding caustic soda to the mixture. |
Litmus turns blue |
NH4+ May be |
|
4. |
On boiling the mixture with caustic soda and placing the filter paper moist with mercurous nitrate. |
Filter paper turns black |
NH4+ Conformed |
|
5. |
Boil the mixture in aqueous medium and add Nessler’s reagent K2[HgI4] |
Brown precipitate is formed |
NH4+ Conformed |
Flame Test :- theoritically few compound an volatile, specially metal chlorides which are none volatile and get ionise on heating. These ionised cation give specific colour on heating in flame, on the basis of which protable cation can be estimated.
For flame Test, we require platimium wire, which is dipped in dil. HCl and heat in the flame till into colour in flame is observer.
Now make a paste of conc. HCl and salt in a watch glass, adn dip the tip of platinum wire in two paste and take, it to the flame and observe the change in colour.
Chemistry of flame test: This is based on the fact that characteristic colours to the flame. We know that metal chlorids are generally more volatile hence substance is moistened to convert the basic readicals into their chlorides.
Borex Bead test: -theory :- it is one of the oldest method which was introduced by berzelins in 1812. since then other salts were used as flexing agents, like sodium carbonate or sodium floride. the most importent one after borex is microcosmic salt, which is the basis of microcosmic salt bead test.
in this test borex Na2B4O7.H2O is heated in the loop of pt wire, if swalls and formn transparent colourless glassy bead when this hot bead is touched with small amount of coloured salt and characteristic colour.
Following is the table which shows different colours in different element-
|
Element |
Oxidising flame |
Redicing flame |
|
Hot |
Cold |
Hot/ColdgreenColorlessgrecyRed-brownBlueGreen |
|
Chrome |
yellow-red |
green |
|
manganess |
Violet |
Violet |
|
Nickel |
Brown |
Red-brown |
|
Copper |
Green |
Green-blue |
|
Cobalt |
Blue |
Blue |
|
Iron |
Yellow-red |
Yellow-colours |
Charcoal cavity test – It is perfomed by mixing the given salt with sodium carbonate and heating the mixture on the block of charcoal with the help of blow pipe in reducing flame. The salt gets converted to respective oxides having charecteristies colours.
Procedure- Take a charcoal box with a small cavity in it. Take a small amount of salt in a watch glass, mix it with solid sodium carbonate whose quantity is 2- times that of the salt. Put this mixture in the cavity.
Cobalt Nitrate Ash test – Dip a dry filter in solution of cobalt nitrate adn dry it. The flame slowly, then ignite this dyr litmus paper to make ash of it, check and observe the colour of ash produced to detect the presence of cation using following table.
|
Colour of ash |
Cation |
|
1. Green |
Zn2+ |
|
2.Blue |
Al3+ |
|
3. Pink |
Mg2+ |
|
4. Dirty Blue |
Sn2+ or Sn4+ |
Note – Don’t use excess of cobalt nitrate solution otherwise it will give Co3O4 of black colour in this condition other colours are not visible.

