SOLUTIONS
Q1. If 1.202 g mL-1 is the density of 20% aqueous KI, determine the following:
(a) Molality of KI (b) Molarity of KI (c) Mole fraction of KI
Ans. 20% solution of KI means 20g of KI are present in 100g of solution or 80g of water. (a) Molar mass of KI = 166
Moles of KI = 20/166 = 0.120
Molality = 0.120 x 1000 /80 = 1.5 m
- Volume of solution = 100/1.202 = 83.19 ml
Molarity = 0.120 x 1000 / 83.19 = 1.44 M
- Moles of KI = 20/166 = 0.120
Moles of water = 80/18 = 4.44
Mole fraction of KI 0.120/ (4.44+0.120) = 0.0263
Q2. Calculate Henry’s law constant when the solubility of H2S (a toxic gas with rotten egg like smell) in water at STP is 0.195 m
Ans. Moles of H2S = 0.195
Moles of water = 1000/18 = 55.55
Mole fraction of H2S = 0.195 / (55.55+0.195) = 0.0035
p = KH x2
KH = p / x2 = 0.987 bar / 0.0035 = 282 bar.
Q3. 18g of glucose, C6H12O6, is dissolved in 1 kg of water in a sauce pan. At what temperature will the water boil. Kb for water is 0.52 K kg mol-1and boiling point of water is 373.15 K.
Ans. ∆Tb = Kb m = (Kb x wB x 1000)/MB x wA
(0.52 x 18 x 1000) / (180 x 1000) = 0.052
boiling point of the solution = 373.15 + 0.052 = 373.202 K
Q4. 2 g of benzoic acid (C6H5COOH) dissolved in 25 g of benzene shows a depression in freezing point equal to 1.62 K. Molal depression constant for benzene is 4.9 K kg mol–1. What is the percentage association of acid if it forms dimer in solution?
Ans. ∆Tf = Kf m = (Kf x wB x 1000)/MB x wA
MB = (4.9 x 2 x 1000) / (1.62 x 25) = 241.98 gmol-1
Molar mass of C6H5COOH = 122 gmol-1
2C6H5COOH ⇌ C6H5COOH)2
Initial moles 1 0
Moles after association (1- α) α/2
Total moles after association = 1- α + α/2 = 1- α/2
i = Normal molar mass/ Observed molar mass = 122 / 241.98 = 0.504
i = (1- α/2) / 1 = 0.504
α = 0.992
Thus, the degree of association of benzoic acid in benzene = 99.2%
Q5. Determine the osmotic pressure of a solution prepared by dissolving 25 mg of K2SO4 in 2 litres of water at 25° C, assuming that it is completely dissociated.
Ans. If K2SO4 is completely dissociated, then i = 3
Molar mass of K2SO4 = 174
π= i cRT= i (nB/V) RT = i (wB RT/MBV) = 3 x 25 x10-3 x 0.082 x 298 / 174 x 2
π = 5.27 x 10-3 atm.
ELECTROCHEMISTRY
Q1. Λm0 for NaCl, HCl and CH3COONa are 126.4, 425.9 and 91.0 S cm2 mol–1 respectively. Calculate Λm0 for CH3COOH.
Ans: Λm0 (HCl) + Λm0 (CH3COONa) – Λm0 (NaCl)
425.9 + 91.0 – 126.4 = 390.5 S cm2 mol–1
Q2. Calculate Ecell for the reaction given below, if E0cell = 3.17V
Mg (s) + 2Ag+ (0.0001M) →Mg2+ (0.130M) + 2Ag (s)
Ans:
Ecell = E0cell – (0.059/2) log {[Mg2+] / [Ag+]2 }
= 3.17 – 0.0295 log [(0.130)/ (0.0001)2]
= 3.17 V – 0.21V = 2.96 V
Q3. A solution of CuSO4 is electrolysed for 10 minutes with a current of 1.5 amperes. What is the mass of copper deposited at the cathode?
Ans: t = 10 x 60 = 600 s
Q = I X t = 1.5 A × 600 s = 900C
According to the reaction:
Cu2+ (aq) + 2e– = Cu(s)
We require 2F or 2 × 96500 C to deposit 1 mol or 63 g of Cu.
For 900 C, the mass of Cu deposited
= (63 g mol–1 × 900 C)/ (2 × 96500 C mol–1) = 567 / 1930 g = 0.2938 g.
Q4. The electrical resistance of a column of 0.05 mol L–1 NaOH solutions of diameter 1 cm and length 50 cm is 5.55 × 103 ohm. Calculate its resistivity, conductivity and molar conductivity.
Ans:
A = ᴨ r2 = 3.14 × 0.52 cm2 = 0.785 cm2 = 0.785 × 10–4 m2 l = 50 cm = 0.5 m
R = ρ l/ A
ρ (resistivity) = RA/l = [5.55 × 103 ohm x 0.785 cm2] / 50 cm = 87.135 ohm cm Conductivity (κ) = 1/ ρ = [1 / 87.135] S cm-1 = 0.01148 S cm–1
Molar conductivity, Λm = [κ (S cm-1) x 1000 (cm3/L)] / Molarity (mol/L)
= [0.01148 ×1000]/ 0.05
= 229.6 S cm2 mol–1
Q5. Conductivity of 0.00241 M acetic acid is 7.896 x 10-5 S cm-1. Calculate its molar conductivity. If Λ0m for acetic acid is 390.5 S cm2 mol-1. What is the dissociation constant?
Ans: Molar conductivity, Λm = 32.76 S cm2 mol-1
α= 32.76/390.5 = 8.39 x 10-2 Dissociation constant = 1.86 x 10-5
Q6. Explain the following:
- How much charge is required for 1 mol of MnO4– to Mn2+
- How much electricity is required in coulomb for the oxidation of 1 mol of H2O to O2
- How much electricity in terms of Faraday is required to produce 40g of Al from molten Al2O3
Ans. 1. Reduction of 1 mol of MnO4– requires = 5 x 96500 C
- Oxidation of 1 mol of H2O requires = 2 x 96500 C
- Al3+ + 3e– → Al
1 mol of Al requires 3 mol of electrons or 3 F
27g of Al requires = 3F
40g of Al requires = (3 x 40) / 27 = 4.44F
Q7. The standard electrode potential for Daniell cell is 1.1V. Calculate the standard Gibbs energy for the reaction: Zn(s) + Cu2+ (aq) → Zn2+ (aq) + Cu(s)
Ans: ∆rG0 = – n F E0cell
The value of n in the above equation is 2, F = 96500 C mol–1 and E0cell = 1.1 V
Therefore, ∆rG0 = – 2 × 1.1V × 96500 C mol–1
= – 212300 J mol–1
= – 212.3 kJ mol–1
Q8. Calculate the equilibrium constant of the reaction:
Cu(s) + 2Ag+(aq) ![]() Cu2+(aq) + 2Ag(s)
Cu2+(aq) + 2Ag(s)
E0cell = E0Ag+/Ag – E0Cu2+/Cu = 0.80 – 0.34 = 0.46 V
Ans. log Kc = (2 x 0.46)/0.059 = 15.59
Kc = 3.92 × 1015
Q9. The standard electrode potential for Daniell cell is 1.1V. Calculate the standard Gibbs energy for the reaction: Zn(s) + Cu2+ (aq) → Zn2+ (aq) + Cu(s)
Ans: ∆rG0 = – n F E0cell
The value of n in the above equation is 2, F = 96500 C mol–1 and E0cell = 1.1 V
Therefore, ∆rG0 = – 2 × 1.1V × 96500 C mol–1
∆rG0 = – 212300 J mol–1
∆rG0 = – 212.3 kJ mol–1
Q10. Write the cell reaction of a lead storage battery when it is discharged. How does the density of the electrolyte change when the battery is discharged?
Ans. Pb + PbSO4 + 2H2SO4 → 2PbSO4 + 2H2O
Density of electrolyte decreases because water is formed and sulphuric acid is consumed as the product during discharge of the battery.
CHEMICAL KINETICS
Q1. For the reaction R ![]() P, the concentration of a reactant changes from 0.03M to 0.02M in 25 minutes. Calculate the average rate of reaction using units of time both in minutes and seconds.
P, the concentration of a reactant changes from 0.03M to 0.02M in 25 minutes. Calculate the average rate of reaction using units of time both in minutes and seconds.
Ans. Average rate = 4 x 10-4 mol L-1 min-1
Rate = 6.67 x 10-6 mol L-1 s-1
Q2. Calculate the overall order of a reaction which has the rate expression:
(a) Rate = k [A]1/2 [B]3/2
(b) Rate = k [A]3/2 [B]–1
Ans. (a) Second order
(b) half order.
Q3. Identify the reaction order from each of the following rate constants.
- k = 2.3 × 10–5 L mol–1 s–1
- k = 3 × 10–4 s–1
Ans. (i) Second order reaction
(ii) first order reaction.
Q4. For a reaction, A + B ![]() Product; the rate law is given by, r = k [A]1/2 [B]2.
Product; the rate law is given by, r = k [A]1/2 [B]2.
What is the order of the reaction?
Ans. Order = 2.5
Q5. The initial concentration of N2O5 in the following first order reaction
N2O5 (g) ![]() 2 NO2 (g) + 1/2O2 (g) was 1.24 × 10–2 mol L–1 at 318 K. The concentration of N2O5 after 60 minutes was 0.20 × 10–2 mol L–1.
2 NO2 (g) + 1/2O2 (g) was 1.24 × 10–2 mol L–1 at 318 K. The concentration of N2O5 after 60 minutes was 0.20 × 10–2 mol L–1.
Calculate the rate constant of the reaction at 318 K.
Ans: k = 0.0304 min-1
Q6. The rate constant for a first order reaction is 60 s–1. How much time will it take to reduce the initial concentration of the reactant to its 1/16th value?
Ans. t = 2.303/k {log[A]0/[A]}
= 2.303/60 {log (a/a/16)}
= 0.038 log16
= 0.038 x 1.204
= 0.046 seconds
Q7. A first order reaction takes 40 min for 30% decomposition. Calculate t1/2.
Ans. k = 2.303/t {log[A]0/[A]}
=2.303/40{log(a/0.70a)} = 2.303/40 {0.1549} k = 8.92 x 10-3 min-1
t1/2 = 0.693/k t1/2 = 0.693/8.92 x 10-3 min-1
t1/2 = 77.7 min.
Q8. The rate of the chemical reaction doubles for an increase of 10K in absolute temperature from 298K. Calculate Ea.
Ans. log k2 – log k1 = Ea/2.303 R [1/T1 – 1/T2]
logk2/k1 = Ea/2.303 R [1/T1 – 1/T2]
log2 = (Ea/2.303 x 8.314) [1/298 – 1/308]
Ea = log2 x 2.303 x 8.314 x 298 x 308
10
Ea = 52.898 kJ
d-AND f-BLOCK ELEMENTS
Q1. Why is Cr2+ reducing and Mn3+ oxidising when both have d4 configuration?
Ans. Cr2+ is reducing as its configuration changes from d4 to d3, the latter having a half-filled t2g level. On the other hand, the change from Mn3+ to Mn2+ results in the half-filled (d5) configuration which has extra stability.
Q2. (i) Explain why Cu+ ion is not stable in aqueous solutions?
(ii) How would you account for the increasing oxidising power in the series?
VO2+ < Cr2O72– < MnO4–?
Ans. (i) because less negative enthalpy of hydration than of Cu2+.
(ii) This is due to the increasing stability of the lower species to which they are reduced.
Q3. Why is the E0 value for the Mn3+/Mn2+ couple much more positive than that for Cr3+/Cr2+ or Fe3+/Fe2+? Explain.
Ans. Much larger third ionization energy of Mn (where the required change is d5 to d4) is mainly responsible for this.
Q4. Calculate the magnetic moment of a divalent ion in aqueous solution if its atomic number is 25.
Ans. With atomic number 25, the divalent ion in aqueous solution will have d5 configuration (five unpaired electrons). The magnetic moment, μ is μ = 5(5 + 2) = 5.92 BM
Q5. What is meant by ‘disproportionation’ of an oxidation state? Give an example.
Ans. When a particular oxidation state becomes less stable relative to other oxidation states, one lower, one higher, it is said to undergo disproportionation.
For example, manganese (VI) becomes unstable relative to manganese (VII) and manganese (IV) in acidic solution.
3 MnVIO4 2– + 4 H+ ![]() 2 MnVIIO4– + MnIVO2 + 2H2O
2 MnVIIO4– + MnIVO2 + 2H2O
Q6. What are interstitial compounds? Why are such compounds well known for transition metals?
Ans. The small atoms (H, B, C, N) get trapped in vacant spaces of the lattices of the transition metal atoms.
Q7. (i) Name a member of the lanthanoids series which is well known to exhibit +4 oxidation state.
(ii) Actinoids contraction is greater from element to element than lanthanoids contraction. Why?
(iii) Which out of Lu (OH)3 and La (OH)3 is more basic and why?
Ans. (i) Cerium (Z = 58)
- This is because of poor shielding by 5f electrons in actinoids in comparison with shielding of 4f electrons in lanthanoids.
- La (OH)3 is more basic than Lu (OH)3 due to lanthanoids contraction.
Q8. (a)Indicate the steps in the preparation of K2Cr2O7 from chromite ore.
- What is the effect of increasing pH on a solution of potassium dichromate?
- Describe the oxidising action of potassium dichromate and write the ionic equations for its reaction with:
(i) Iodide (ii) iron (II) solution and (iii) H2S Write the ionic equations for the reactions.
Ans. (a) Give preparation
- Cr2O7 2- + H2O ⇌ 2CrO4 2- + 2H+
- Give equations
Q9. (a)Indicate the steps in the preparation of KMnO4 from pyrolusite ore.
(b) Describe the preparation of potassium permanganate. How does the acidified permanganate solution react with (i) iron (II) ions (ii) SO2 and (iii) oxalic acid?
Write the ionic equations for the reactions.
Ans. (a)
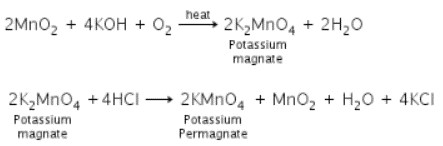
(b)

Q10. Explain giving reasons:
- Transition metals and many of their compounds show paramagnetic behaviour.
- The enthalpies of atomization of the transition metals are high.
- The transition metals generally form coloured compounds.
(iv)Transition metals and their many compounds act as good catalyst.
(v) Scandium (Z = 21) is a transition element but zinc (Z = 30) is not?
Ans. (i) due to unpaired electrons.
- Because of large number of unpaired electrons in their atoms they have stronger interatomic interaction and hence stronger bonding between atoms resulting in higher enthalpies of atomization.
- d-d transition
- Due to the presence of vacant orbitals or their tendency to form variable oxidation state.
- On the basis of incompletely filled 3d orbitals in case of scandium atom in its ground state (3d1), it is regarded as a transition element. On the other hand, zinc atom has completely filled d orbitals (3d10) in its ground state as well as in its oxidised state; hence it is not regarded as a transition element.
COORDINATION COMPOUNDS
Q1. Write the formulas for the following coordination compounds:
- Tetraammineaquachloridocobalt (III) chloride
- Potassium tetrahydroxidozincate (II)
- Potassium trioxalatoaluminate (III)
- Dichloridobis (ethane-1, 2-diamine) cobalt (III) ion
- Tetracarbonyl nickel (0)
Ans: (a) [Co (NH3)4(H2O) Cl] Cl2
(b) K2 [Zn (OH)4]
(c) K3 [Al (C2O4)3]
- [Co Cl2 (en)2] +
- [Ni (CO)4]
Q2. Write the IUPAC names of the following coordination compounds:
- [Pt (NH3)2Cl (NO2)]
- K3 [Cr (C2O4)3]
- [Co Cl2 (en)2] Cl
- [Co (NH3)5(CO3)] Cl
- Hg [Co (SCN)4]
Ans:
- Diamminechloridonitrito-N-platinum (II)
- Potassium trioxalatochromate (III)
- Dichloridobis (ethane-1, 2-diamine) cobalt (III) chloride
- Pentaamminecarbonatocobalt (III) chloride
- Mercury (I) tetrathiocyanato-S-cobaltate (III)
Q3. Draw structures of geometrical isomers of [Fe (NH3)2(CN)4]–
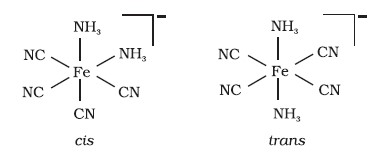
Q4. Indicate the types of isomerism exhibited by the following complexes and draw the structures for these isomers:
- K [Cr (H2O)2(C2O4)2
- [Co(en)3] Cl3
- [Co (NH3)5(NO2)] (NO3)2
- [Pt (NH3) (H2O) Cl2]
Ans. (i) Both geometrical (cis and trans) and optical isomers for cis.
- Two optical isomers
- Geometrical, ionization and linkage isomers.
- Geometrical (cis and trans)
Q5. Give evidence that [Co (NH3)5Cl] SO4 and [Co (NH3)5(SO4)] Cl are ionization isomers.
Ans. [Co (NH3)5Cl] SO4 + BaCl2 ![]() BaSO4 (white ppt)
BaSO4 (white ppt)
[Co (NH3)5(SO4)] Cl + AgNO3 ![]() AgCl (white ppt)
AgCl (white ppt)
Q6. The spin only magnetic moment of [MnBr4]2– is 5.9 BM. Predict the geometry of the complex ion?
Ans. Since the coordination number of Mn2+ ion in the complex ion is 4, it will be either tetrahedral (sp3 hybridization) or square planar (dsp2 hybridization). But the fact that the magnetic moment of the complex ion is 5.9 BM, it should be tetrahedral in shape rather than square planar because of the presence of five unpaired electrons in the d orbitals.
Q7. Predict the number of unpaired electrons in the square planar [Pt (CN)4]2– ion.
Ans. Pt = 5d9 6s1
Pt (II) = 5d8, square planar geometry and dsp2 hybridization.
Q8. Explain on the basis of valence bond theory that [Ni (CN)4]2– ion with square planar structure is diamagnetic and the [NiCl4]2– ion with tetrahedral geometry is paramagnetic.
Ans. [Ni (CN)4]2– square planar geometry and dsp2 hybridization.
[NiCl4]2– Tetrahedral geometry and sp3 hybridization.
Q9. [Fe (H2O)6]3+ is strongly paramagnetic whereas [Fe (CN)6]3– is weakly paramagnetic. Explain.
Ans. In both the complexes, Fe is in +3 oxidation state.
CN– is strong field ligand, inner d-orbitals are involved, d2sp3 hybridization, one unpaired electron, weakly paramagnetic.
H2O is weak field ligand, outer d-orbitals are involved, sp3d2 hybridization, five unpaired electrons, strongly paramagnetic.
Q10. Explain [Co (NH3)6]3+ is an inner orbital complex whereas [Ni (NH3)6]2+ is an outer orbital complex.
Ans. [Co (NH3)6]3+ is an inner orbital complex, d2sp3 hybridization.
[Ni (NH3)6]2+ is an outer orbital complex, sp3d2 hybridization.
HALOALKANES AND HALOARENES
Q1. Draw the structures of all the eight structural isomers that have the molecular formula C5H11Br. Name each isomer according to IUPAC system and classify them as primary, secondary or tertiary bromide.
Ans. CH3CH2CH2CH2CH2Br 1-Bromopentane (primary)
CH3CH2CH2CH (Br) CH3 2-Bromopentane (secondary)
CH3CH2CH (Br) CH2 CH3 3-Bromopentane (secondary)
(CH3)2CHCH2CH2Br 1-Bromo-3-methylbutane (primary)
(CH3)2CHCHBrCH3 2-Bromo-3-methylbutane (secondary)
(CH3)2CBrCH2CH3 2-Bromo-2-methylbutane (tertiary)
CH3CH2CH (CH3) CH2Br 1-Bromo-2-methylbutane (primary)
(CH3)3CCH2Br 1-Bromo-2, 2-dimethylpropane (primary)
Q2. Write IUPAC names of the following:
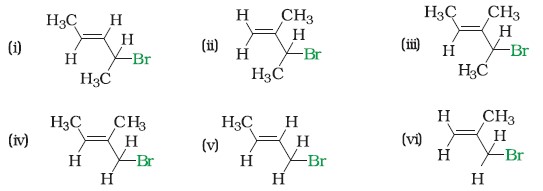
Ans.
- 4-Bromopent-2-ene
- 3-Bromo-2-methylbut-1-ene
- 4-Bromo-3-methylpent-2-ene
- 1-Bromo-2-methylbut-2-ene
- 1-Bromobut-2-ene
- 3-Bromo-2-methylpropene
Q3. Why is Sulphuric acid not used during the reaction of alcohols with KI?
Ans. Sulphuric acid is an oxidising agent. It will oxidise HI produced during the reaction to I2 and therefore, will prevent reaction between an alcohol and HI to form alkyl halide.
Q4. Write structures of different dihalogen derivatives of propane.
Ans. 1,1-dibromopropane, 1,2-dibromopropane, 1,3-dibromopropane, 2,2-dibromopropane
Q5. Arrange each set of compounds in order of increasing boiling points.
- Bromomethane, Bromoform, Chloromethane, Dibromomethane.
- 1-Chloropropane, Isopropyl chloride, 1-Chlorobutane.
Ans. (i) Chloromethane < Bromomethane < Dibromomethane < Bromoform (ii) Isopropyl chloride < 1-Chloropropane < 1-Chlorobutane
Q6. Haloalkanes react with KCN to form alkyl cyanides as main product while AgCN forms isocyanides as the chief product. Explain.
Ans. KCN is predominantly ionic and provides cyanide ions in solution. Although both carbon and nitrogen atoms are in a position to donate electron pairs, the attack takes place mainly through carbon atom and not through nitrogen atom since C—C bond is more stable than C—N bond. However, AgCN is mainly covalent in nature and nitrogen is free to donate electron pair forming isocyanide as the main product.
Q7. In the following pairs of halogen compounds, which would undergo SN2 reaction faster?
- C6H11-CH2Cl and C6H11-Cl
- CH3CH2CH2CH2I and CH3CH2CH2CH2Cl
Ans. (i) C6H11-CH2Cl is primary halide and therefore undergoes SN2 reaction faster.
(ii) CH3CH2CH2CH2I will undergoes SN2 reaction faster because iodide ion is a better leaving group because of its large size, it will be released at a faster rate in the presence of incoming nucleophile.
Q8. Predict the order of reactivity of the following compounds in SN1 and SN2 reactions:
- The four isomeric bromobutanes
- C6H5CH2Br, C6H5CH (C6H5) Br, C6H5 CH (CH3) Br, C6H5 C (CH3) (C6H5) Br
Ans. In SN1 reaction, stability of carbocation decreases, tertiary > secondary > primary
- CH3CH2CH2CH2Br < (CH3)2CHCH2Br < CH3CH2CH (Br) CH3 < (CH3)3CBr (SN1)
CH3CH2CH2CH2Br > (CH3)2CHCH2Br > CH3 CH2 CH (Br) CH3 > (CH3)3CBr (SN2)
- C6H5 C (CH3) (C6H5) Br > C6H5 CH (C6H5) Br > C6H5 CH (CH3) Br > C6H5CH2Br (SN1)
C6H5 C (CH3) (C6H5) Br < C6H5 CH (C6H5) Br < C6H5 CH (CH3) Br < C6H5CH2Br (SN2)
Q9. Explain why (i) the dipole moment of Chlorobenzene is lower than that of cyclohexyl chloride?
- Alkyl halides, though polar, are immiscible with water?
- Grignard reagents should be prepared under anhydrous conditions?
Ans. (i) Due to magnitude of negative charge (δ-) is less on Cl atom of Chlorobenzene than in cyclohexyl chloride.
- Alkyl halides, though polar, are immiscible with water because the molecules of water are held together by hydrogen bonds.
- Grignard reagents are very reactive.
Q10. Primary alkyl halide C4H9Br (a) reacted with alcoholic KOH to give compound (b). Compound (b) is reacted with HBr to give (c) which is an isomer of (a). When (a) is reacted with sodium metal it gives compound (d), C8H18 which is different from the compound formed when n-butyl bromide is reacted with sodium. Give the structural formula of (a) and write the equations for all the reactions.
Ans. (a) isobutyl bromide
- 2-methyl prop-1-ene
- t-butyl bromide
- 2, 5-dimethyl hexane.
ALCOHOLS, PHENOLS AND ETHERS
Q1. Give the structures and IUPAC names of the products expected from the following reactions:
- Catalytic reduction of butanal.
- Hydration of propene in the presence of dilute Sulphuric acid.
- Reaction of Propanone with methyl magnesium bromide followed by hydrolysis.
Ans. (a) Butan-1-ol
- Propan-2-ol
- 2-methyl Propan-2-ol
Q2. Arrange the following sets of compounds in order of their increasing boiling points:
- Pentan-1-ol, butan-1-ol, butan-2-ol, ethanol, propan-1-ol, methanol.
- Pentan-1-ol, n-butane, pentanal, ethoxyethane.
Ans. (a) Methanol, ethanol, propan-1-ol, butan-2-ol, butan-1-ol, pentan-1-ol.
(b) n-Butane, ethoxyethane, pentanal and pentan-1-ol.
Q3. Arrange the following compounds in increasing order of their acid strength:
Propan-1-ol, 2, 4, 6-trinitrophenol, 3-nitrophenol, 3, 5-dinitrophenol, phenol, 4-methylphenol.
Ans. Propan-1-ol, 4-methylphenol, phenol, 3-nitrophenol, 3,5-dinitrophenol, 2,4, 6-trinitrophenol.
Q4. Give structures of the products you would expect when each of the following alcohol reacts with (a) HCl –ZnCl2 (b) HBr and (c) SOCl2.
- Butan-1-ol
- 2-Methylbutan-2-ol
Ans. (a) HCl –ZnCl2, it is a Lucas reagent.
(i) Turbidity appears upon heating (ii) white turbidity formed
(b) With HBr to give alkyl halides.
- Both alcohols react to give alkyl chlorides.
Q5. Predict the major product of acid catalyzed dehydration of
- 1-methylcyclohexanol (ii) butan-1-ol
Ans. (i) 1- Methyl cyclohexene (ii) But-2-ene.
Q6. Ortho and para nitrophenols are more acidic than phenol. Draw the resonance structures of the corresponding phenoxide ions.
Ans. Draw the resonance structures of o- & p- phenoxide ions.
Due to –I and –R effect of –NO2 group, o- & p- phenoxide ions are more stable. Hence, Ortho and para nitrophenol are more acidic than phenol.
Q7. Write the equations involved in the following reactions:
- Reimer – Tiemann reaction
- Kolbe’s reaction
Ans. (i) Reimer – Tiemann reaction – when phenol reacts with chloroform in the presence of aqueous sodium hydroxide, salicylaldehyde is formed.
(ii) Kolbe’s reaction- when sodium phenoxide is heated with carbon dioxide at about 400K and under
4 to 7 atmospheric pressure, sodium salicylate is formed. On acidification gives salicylic acid.
Q8. The following is not an appropriate reaction for the preparation of t-butyl ethyl ether.
C2H5Na + (CH3)3 – Cl ![]() (CH3)3 – OC2H5
(CH3)3 – OC2H5
- What would be the major product of this reaction?
- Write a suitable reaction for the preparation of t-butyl ethyl ether.
Ans. (i) The major product of the given reaction is 2-methylprop-1-ene. It is because sodium ethoxide is a strong nucleophile as well as a strong base. Thus, elimination reaction predominates over substitution.
- (CH3)3 – O– Na+ + CH3CH2Cl
 (CH3)3 – OC2H5
(CH3)3 – OC2H5
Q9. Write the mechanism of hydration of Ethene to yield ethanol.
Ans. It is an example of electrophilic addition.
CH2 = CH2 + H+ ![]() CH3CH2OH
CH3CH2OH
Q10. Write the names of reagents and equations for the preparation of the following ethers by Williamson’s synthesis:
- 1-Propoxypropane
- Ethoxy benzene
- 2-Methoxy-2-methylpropane
- 1-Methoxyethane
Ans. (i) Sodium propoxide + 1-bromopropane
(ii) Sodium phenoxide + ethyl bromide
(iii)Sodium 2-methyl-2-propoxide + Bromomethane
(iv)Sodium ethoxide + Bromomethane
ALDEHYDES, KETONES AND CARBOXYLIC ACIDS
Q1. Name the following compounds according to IUPAC system of nomenclature:
- CH3CH(CH3) CH2CH2CHO
- CH3CH2COCH(C2H5) CH2CH2Cl
- CH3CH=CHCHO
- CH3COCH2COCH3
- CH3CH(CH3) CH2C(CH3)2COCH3
- (CH3)3CCH2COOH
- OHCC6H4CHO-p
Ans. (i) 4-methyl pentanal
- 6-chloro-4-ethyl hexan-3-one
- But-2-enal
- Pentane-2,4-dione
- 3,3,5-trimethyl hexane-2-one
- 3,3-dimethyl butanoic acid
- Benzene-1,4-dicarbaldehyde
Q7. Give names of the reagents to bring about the following transformations:
- Hexan-1-ol to hexanal
- Cyclohexanol to cyclohexanone
- p-Fluor toluene to p-Fluoro benzaldehyde
- Ethanenitrile to ethanal
- Allyl alcohol to propenal
- But-2-ene to ethanal
Ans.
- C5H5NH+CrO3Cl– (PCC)
- Anhydrous CrO3 or K2Cr2O7 in acidic medium
- CrO3 in the presence of acetic anhydride or 1. CrO2Cl2 2. H2O
- (Di isobutyl) Aluminium hydride (DIBAL-H)
- PCC
- O3 /H2O-Zn dust
Q2. Arrange the following compounds in the increasing order of their boiling points: CH3CH2CH2CHO, CH3CH2CH2CH2OH, H5C2 -O-C2H5 and CH3CH2CH2CH3
Ans. Increasing order of boiling points of the given compounds is as follows:
CH3CH2CH2CH3 < H5C2 -O-C2H5 < CH3CH2CH2CHO < CH3CH2CH2CH2OH
Q3. Although phenoxide ion has a greater number of resonating structures than carboxylate ion, carboxylic acid is a stronger acid than phenol. Why?
Ans. The electronic charge in the carboxylate ion is more dispersed in comparison to phenate ion.
Carboxylate ion is more stable as compared to phenate ion. The release of H+ ion is easier from carboxylic acid. It behaves as stronger acid than phenol.
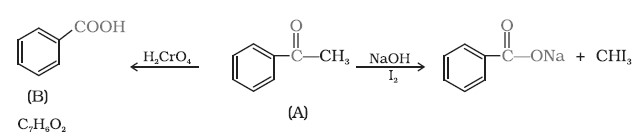
Q4. An organic compound (A) with molecular formula C8H8O forms an orange-red precipitate with 2,4DNP reagent and gives yellow precipitate on heating with iodine in the presence of sodium hydroxide. It neither reduces Tollens’ or Fehling’s reagent, nor does it decolourise bromine water or Baeyer’s reagent. On drastic oxidation with chromic acid, it gives a carboxylic acid (B) having molecular formula C7H6O2.
Identify the compounds (A) and (B) and explain the reactions involved.
Ans. Reactions are as follows:
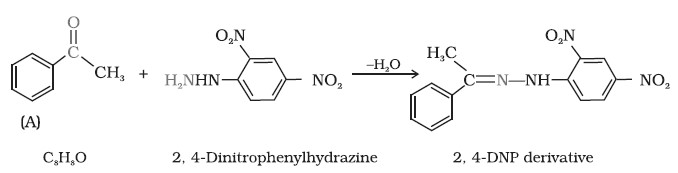
Q5. Arrange the following compounds in increasing order of their reactivity in nucleophilic addition reactions.
- Ethanal, Propanal, Propanone, Butanone.
- Benzaldehyde, p-Tolualdehyde, p-Nitro benzaldehyde, Acetophenone.
Ans. (i) Butanone < Propanone < Propanal < Ethanal
(ii) Acetophenone < p-Tolualdehyde < Benzaldehyde < p-Nitro benzaldehyde
Q6. An organic compound with the molecular formula C9H10O forms 2, 4-DNP derivative, reduces
Tollens’ reagent and undergoes Cannizzaro reaction. On vigorous oxidation, it gives 1,2benzenedicarboxylic acid. Identify the compound.
Ans. 2-Ethyl benzaldehyde
Q7. An organic compound (A) (molecular formula C8H16O2) was hydrolyzed with dilute Sulphuric acid to give a carboxylic acid (B) and an alcohol (C). Oxidation of (C) with chromic acid produced (B). (C) on dehydration gives but-1-ene. Write equations for the reactions involved. Ans. A = Butyl butanoate, B = Butanoic acid, C = Butanol
Q8. Arrange the following compounds in increasing order of their property as indicated:
- Acetaldehyde, Acetone, Di-tert-butyl ketone, Methyl tert-butyl ketone (reactivity towards HCN)
- CH3CH2CH(Br)COOH, CH3CH(Br)CH2COOH, (CH3)2CHCOOH, CH3CH2CH2COOH (acid strength)
- Benzoic acid, 4-Nitrobenzoic acid, 3,4-Dinitrobenzoic acid, 4-Methoxybenzoic acid (acid strength)
Ans. (i) Acetaldehyde > Acetone > Methyl tert-butyl ketone > Di-tert-butyl ketone
- (CH3)2CHCOOH < CH3CH2CH2COOH < CH3 CH (Br) CH2 COOH < CH3CH2CH (Br) COOH
- 4-Methoxybenzoic acid < Benzoic acid < 4-Nitrobenzoic acid < 3, 4-Dinitrobenzoic acid
Q9. Give plausible explanation for each of the following:
- Cyclohexanone forms cyanohydrin in good yield but 2,2,6-trimethylcyclohexanone does not.
- There are two –NH2 groups in semi carbazide. However, only one is involved in the formation of semi carbazones.
- During the preparation of esters from a carboxylic acid and an alcohol in the presence of an acid catalyst, the water or the ester should be removed as soon as it is formed.
Ans. (i) Cyclohexanone forms cyanohydrin in good yield but 2, 2, 6-trimethylcyclohexanone does not because of presence of three methyl groups at -position w.r.t carbonyl group which hinder the Nucleophilic attack of CN– group due steric hindrance. No such steric hindrance in Cyclohexanone.
(ii) Semi carbazide has two –NH2 groups but one of these which is directly attached to C=O is involved in resonance. Electron density on NH2 group decreases hence it does not act as nucleophile.
(iii)It is a reversible reaction. Therefore, to shift the equilibrium in the forward direction, the water or the ester should be removed as fast as it is formed.
AMINES
Q1. Write the names according to IUPAC and indicate primary, secondary and tertiary amines of different isomeric amines corresponding to the molecular formula, C4H11N.
Ans. Eight isomeric amines corresponding to the molecular formula, C4H11N:
- Butanamine (primary)
- Butan-2-amine(primary)
- 2-methyl propanamine (primary)
- 2-methyl propan-2-amine (primary)
- N-methyl propanamine (secondary)
- N-methyl propan-2-amine (secondary)
- N-ethyl Ethanamine (secondary)
- N, N-dimethyl Ethanamine (tertiary)
Q2. Write chemical equations for the following reactions:
- Reaction of ethanolic NH3 with C2H5Cl.
- Ammonolysis of benzyl chloride and reaction of amine so formed with two moles of CH3Cl.
Ans. (i) Ethanamine, N-ethyl Ethanamine, N, N-diethyl Ethanamine is formed.
(ii)Benzyl amine, N, N-dimethyl phenyl methanamine is formed.
Q3. Write chemical equations for the following conversions:
- CH3 –CH2 –Cl into CH3 –CH2 –CH2 –NH2
- C6H5 –CH2 –Cl into C6H5 –CH2 –CH2 –NH2
Ans. By treatment with KCN followed by treatment with Na, C2H5OH
(i)CH3 –CH2 -Cl + KCN ![]() CH3 –CH2 -CN + 4H
CH3 –CH2 -CN + 4H ![]()
CH3 –CH2 -CH2 NH2
(ii) C6H5 –CH2 -Cl + KCN ![]() C6H5 –CH2 -CN + 4H
C6H5 –CH2 -CN + 4H ![]() C6H5 –CH2 -CH2 NH2
C6H5 –CH2 -CH2 NH2
Q4. Write structures and IUPAC names of
- the amide which gives propanamine by Hoffmann bromamide reaction.
- the amine produced by the Hoffmann degradation of benzamide.
Ans. (i) Propanamine contains three carbons. Hence, the amide molecule must contain four carbon atoms. Structure and IUPAC name of the starting amide with four carbon atoms are given below:
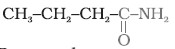
Butanamide
(ii) Benzamide is an aromatic amide containing seven carbon atoms. Hence, the amine formed from benzamide is aromatic primary amine containing six carbon atoms.
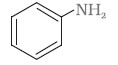
Aniline or benzenamine
Q5. Arrange the following in decreasing order of their basic strength:
C6H5 NH2, C2H5 NH2, (C2H5)2NH, NH3
Ans. The decreasing order of basic strength of the above amines and ammonia follows the following order:
(C2H5)2NH > C2H5NH2 > NH3 > C6H5NH2
Q6. Arrange the following in increasing order of their basic strength:
- C2H5NH2, C6H5NH2, NH3, C6H5CH2NH2 and (C2H5)2NH
- C2H5NH2, (C2H5)2NH, (C2H5)3N, C6H5NH2
- CH3NH2, (CH3)2NH, (CH3)3N, C6H5NH2, C6H5CH2NH2.
Ans. (i) C6H5NH2 < NH3 < C6H5CH2NH2 < C2H5NH2 < (C2H5)2NH
- C6H5NH2 < C2H5NH2 < (C2H5)3N < (C2H5)2NH
- C6H5NH2 < C6H5CH2NH2 < (CH3)3N < CH3NH2 < (CH3)2NH
Q7. (a) Complete the following acid-base reactions and name the products:
- CH3CH2CH2NH2 + HCl →
- (C2H5)3N + HCl →
(b)Write chemical reaction of aniline with benzoyl chloride and write the name of the product obtained.
Ans. (a) (i) CH3CH2CH2NH2 + HCl → CH3CH2CH2NH3+Cl–
(ii) (C2H5)3N + HCl → (C2H5)3NH + Cl–
(b) N-Phenyl benzamide (Benzanilide)
Q8. Arrange the following:
- In decreasing order of the pKb values: C2H5NH2, C6H5NHCH3, (C2H5)2NH and C6H5NH2
- In decreasing order of basic strength: C6H5NH2, C6H5N (CH3)2, (C2H5)2NH and CH3NH2
- In increasing order of basic strength:
- Aniline, p-nitroaniline and p-toluidine
- C6H5NH2, C6H5NHCH3, C6H5CH2NH2.
Ans. (i) Stronger the base lesser the pKb values.
C6H5NH2 > C6H5NHCH3 > C2H5NH2 > (C2H5)2NH
- (C2H5)2NH > CH3NH2 > C6H5N (CH3)2 > C6H5NH2
(iii)(a) p-nitro aniline < Aniline < p-toluidine
(b) C6H5NH2 < C6H5NHCH3 < C6H5CH2NH2.
Q9. An aromatic compound ‘A’ on treatment with aqueous ammonia and heating forms compound ‘B’ which on heating with Br2 and KOH forms a compound ‘C’ of molecular formula C6H7N. Write the structures and IUPAC names of compounds A, B and C.
Ans. A = C6H5COOH, B = C6H5CONH2, C = C6H5NH2.
Q10. Give plausible explanation for each of the following:
- Why are amines less acidic than alcohols of comparable molecular masses?
- Why do primary amines have higher boiling point than tertiary amines?
- Why are aliphatic amines stronger bases than aromatic amines?
- pKb of aniline is more than that of methylamine.
- Aniline does not undergo Friedel-Crafts reaction.
Ans. (i) Loss of proton from amines gives amide ion whereas loss of proton from alcohol gives alkoxide ion. O is more electronegative than N, therefore, alkoxide ion can accommodate the negative charge more easily than amide ion. Alkoxide ion is more stable than amide ion. Thus, alcohols are more acidic than amines.
(ii)Primary amines (RNH2) form intermolecular hydrogen bonding But Tertiary amine (R3N) do not have hydrogen and do not form hydrogen bond.
(iii)Aniline is less basic than ethyl amine by Kb value:
Ethyl amine Kb = 4.7 x 10 -4
Aniline Kb = 4.2 x 10 -10
Aniline is less basic character due to resonance.
(iv) pKb of aniline is higher than that of methyl amine.
(v)Aniline being the Lewis base reacts with Lewis acid AlCl3 to form salt.
C6H5NH2 +AlCl3 ![]() C6H5NH2+AlCl3-
C6H5NH2+AlCl3-
(Salt)
BIOMOLECULES
Q1. Classify the following into monosaccharides and disaccharides.
Ribose, 2-deoxyribose, maltose, galactose, fructose and lactose.
Ans. Ribose, 2-deoxyribose, galactose, fructose – monosaccharides Maltose and lactose – disaccharides.
Q2. Where does the water present in the egg go after boiling the egg?
Ans. When an egg is boiled in water, the water present in egg is used in denaturation of protein probably through H-bonding.
Q3 Why cannot vitamin C be stored in our body?
Ans. Because it is soluble in water and readily excreted in urine and cannot be stored in our body.
Q4. Enumerate the reactions of D-glucose which cannot be explained by its open chain structure.
Ans. (i) Despite having aldehyde group, glucose does not give Schiff test and 2,4-DNP test.
(ii)Glucose does not react with sodium hydrogen bisulphite to form addition product.
(iii)The pentaacetate of glucose does not react with hydroxyl amine showing the absence of free -CHO group.
Q5. What are essential and non-essential amino acids? Give two examples of each type.
Ans. The amino acids which can be made by our bodies and are not required in our diet are called nonessential amino acids. For example, glycine and alanine
The amino acids which cannot be made by our bodies and must be supplied in our diet are called essential amino acids. For example, valine and leucine.
Q6. What happens when D-glucose is treated with the following reagents? (i) HI (ii) Bromine water (iii) HNO3
Ans. (i) when glucose is treated with HI, it forms n-hexane.
- when glucose is treated with Bromine water, gluconic acid is formed.
- when glucose is treated with nitric acid, Saccharic acid is formed.
Q12. Define the following as related to proteins
(i)Peptide linkage (ii) Primary structure (iii) Denaturation.
Ans. (i)Peptide linkage- Peptide bond is formed by the condensation of two or more, same or different α-amino acids. -CO -NH- linkage is called peptide linkage.
- Primary structure- Primary structure of proteins give the sequence in which amino acids are linked in one or more polypeptide chains of proteins.
- Denaturation- A process that changes the physical and biological properties without affecting the chemical composition of a protein is called denaturation. The denaturation is caused by certain physical or chemical treatments such as in pH, temperature, presence of some salts or certain chemical agents.
Q7. (a) How do you explain the amphoteric behaviour of amino acids?
(b)The melting points and solubility in water of amino acids are generally higher than that of the corresponding halo acids. Explain.
Ans. (a) Due to dipolar or Zwitter ion structure, amino acids are amphoteric in nature. The acidic character of the amino acids due to the -NH3+ group and the basic character is due to the -COO– group.
(b)Amino acids have strong electrostatic attraction and hence have high melting points and highly soluble in water.
Q8. Differentiate the following
- Globular and fibrous proteins.
- Nucleoside and a nucleotide.
Ans. (i) Fibrous proteins: They are long and thread like and tend to lie side by side to form fibers. In some cases, they are held together by hydrogen bonds at many points. These proteins serve as a chief structural material of animal tissues. Examples, keratin, collagen
Globular proteins: The molecules of these proteins are folded into compact units and form spheroid shapes. Intermolecular forces are weak. These proteins are soluble in water or aqueous solution of acids, bases or salts. Globular proteins make up all enzymes, hormones, fibrinogen etc. Examples, hemoglobin, insulin
(ii) The nitrogenous base and a pentose sugar are called as nucleosides. The nitrogenous base, a pentose sugar and a phosphate group are called as nucleotides.
Q9. (a) Name the vitamins in each case whose deficiency causes
- Night blindness
- Rickets
- Poor coagulation of blood
- Scurvy
(b)What is isoelectric point?
(c)Which amino acid is not optically active?
Ans. (a) (i) Vitamin A (ii) Vitamin D (iii) Vitamin K (iv) Vitamin C
(b)The pH at which no net migration of amino acid takes place under the influence of an applied electric field is called isoelectric point. For example, isoelectric point of glycine is 6.1.
(c)Glycine, NH2CH2COOH
