- Chemistry
- No Comment
Some Important Name Reactions
Some Important Name Reactions
Rosenmund Reduction:
Acid chloride are converted to corresponding aldehydes by catalytic reduction. The reaction is carried out by passing H2 gas through a hot solution of acid chloride in the presence of Pd deposited over BaSO4 (partially poisoned with sulphur or quinoline).
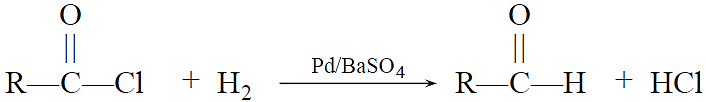
Stephen reaction: Nitriles are reduced to corresponding imines with SnCl2 in the presence of hydrochloric acid, which on hydrolysis give corresponding aldehyde.
SnCl2 + 2HCl→ SnCl4 + 2[H]
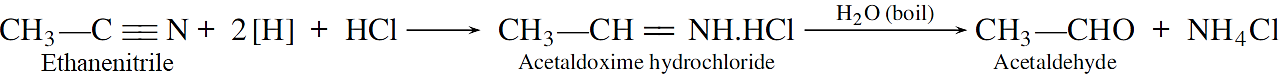
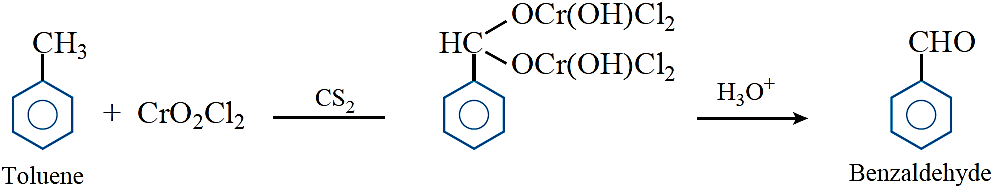
Etard reaction: Chromyl chloride oxidises toluene to chromium complex which on hydrolysis gives Benzaldehyde
Gatterman–Koch reaction: When benzene or its derivative is treated with carbon monoxide and hydrogen chloride in the presence of anhydrous AlCl3 and CuCl, it gives benzaldehyde or substituted benzaldehyde
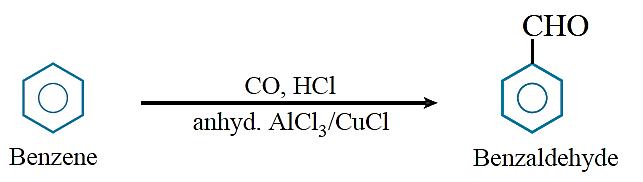
Friedel–Crafts reactions: Friedel–Crafts alkylation: Benzene and other aromatic compounds react with alkyl halides in the presence of anhydrous AlCl3 to form alkyl benzenes.
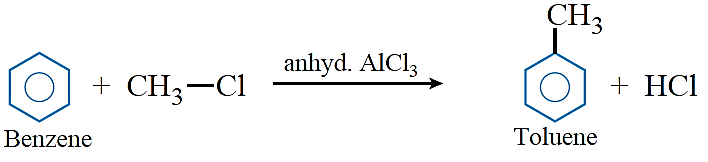
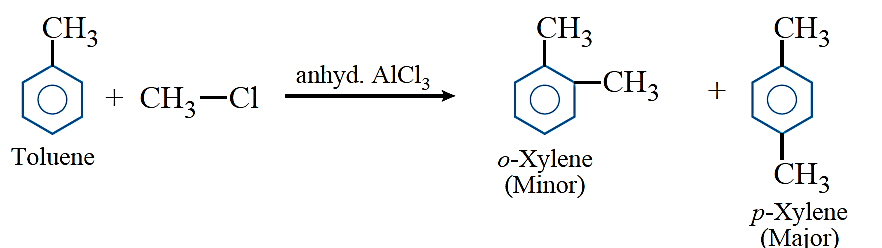
Friedel–Crafts acylation: Benzene and other aromatic compounds react with acylchlorides or acid anhydrides in the presence of anhyd. AlCl3 to form aromatic ketone.
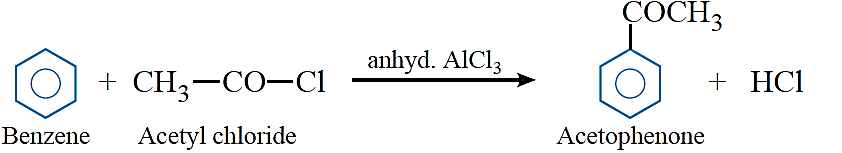
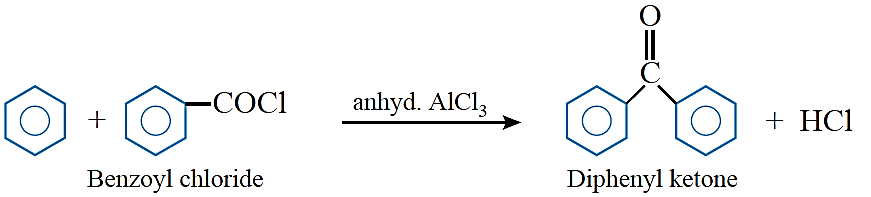
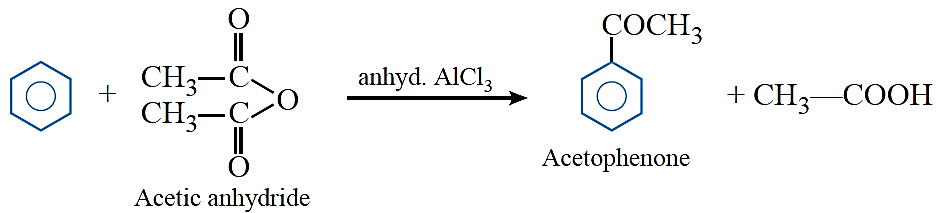
Clemmensen reduction: The carbonyl group of aldehydes and ketones is reduced to CH2 group on treatment with zinc amalgam and concentrated hydrochloric acid.
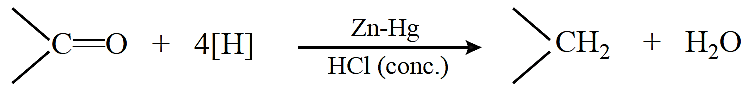
Wolff–Kishner reduction: The carbonyl group of aldehydes and ketones is reduced to —CH2 group on treatment with hydrazine followed by heating with potassium or sodium hydroxide in a high boiling solvent such as ethylene glycol.
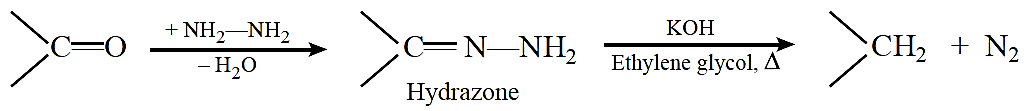
Aldol condensation: Two molecules of aldehydes or ketones containing at least one a-hydrogen atom on treatment with dilute alkali undergo condensation to form b-hydroxy aldehydes (aldol) or b-hydroxy ketones (Ketol).
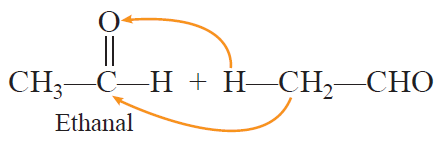
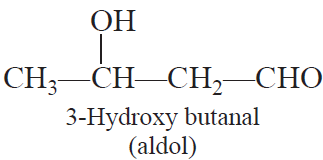
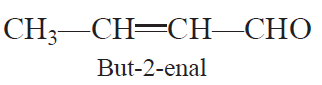
Cross aldol condensation: When aldol condensation is carried out between two different aldehydes and/or ketones, it is called cross aldol condensation
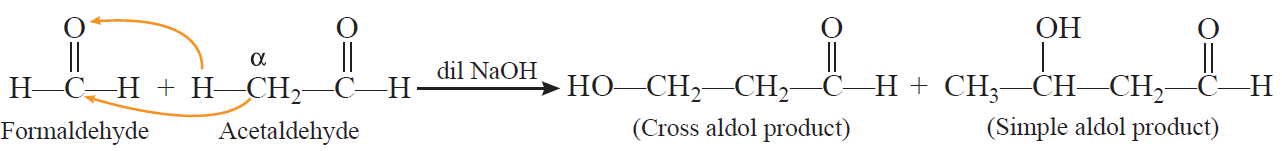
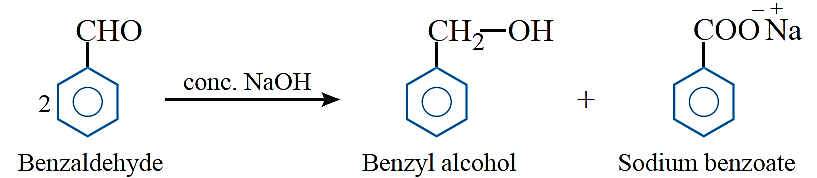
Cannizzaro reaction: Aldehydes which do not have an a-hydrogen, undergo self oxidation and reduction (disproportionation) reaction on treatment with concentrated alkali. In this reaction, one molecule of the aldehyde is reduced to alcohol while another is oxidised to carboxylic acid salt.
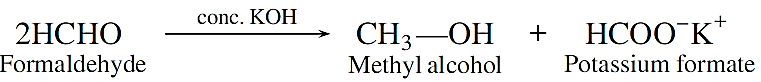
Hell-Volhard-Zelinsky reaction: Carboxylic acids having an a-hydrogen are halogenated at the α-position on treatment with chlorine, or bromine in the presence of red phosphorus to give α -halocarboxylic acids.
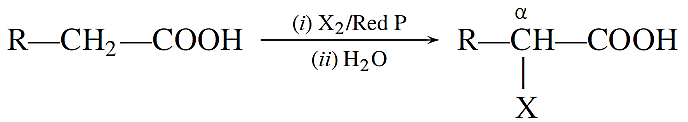
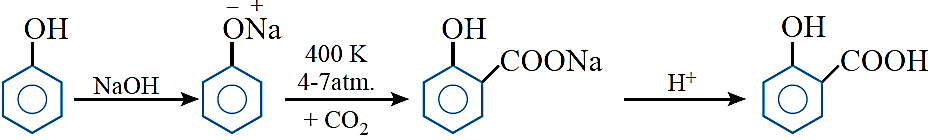
Kolbe’s reaction: When sodium phenoxide is heated with CO2 at 400 K under a pressure of 4 –7 atm, the resulting product on acidification yields salicylic acid.
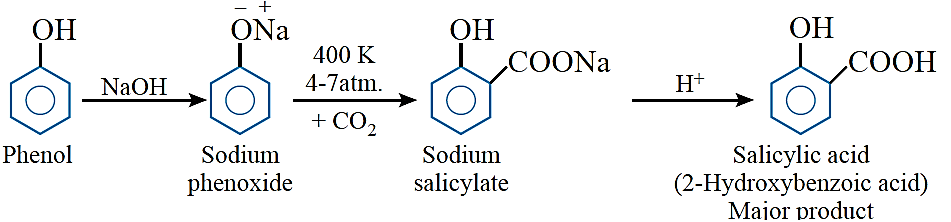
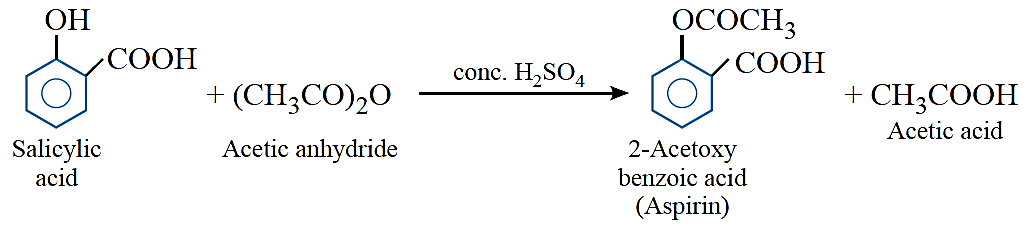
Salicylic acid is the starting material for the manufacture of 2-acetoxybenzoic acid (aspirin).
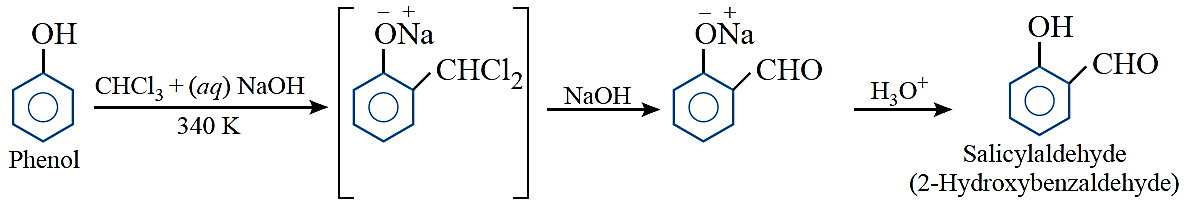
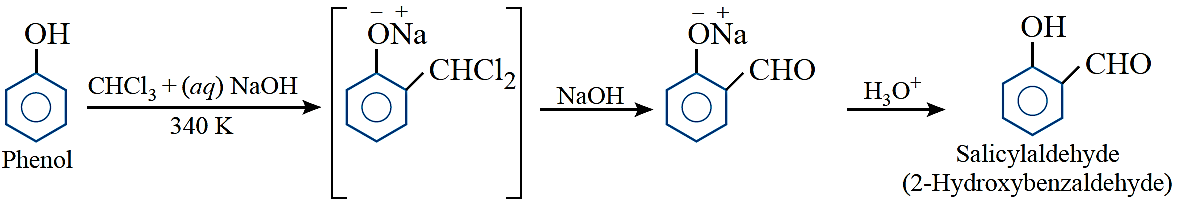
Reimer–Tiemann reaction: Treatment of phenol with chloroform in the presence of sodium hydroxide followed by hydrolysis of resulting product gives o-hydroxybenzaldehyde (salicylaldehyde) as a major product.
Williamson synthesis: It consists of reacting an alkyl halide with sodium alkoxide or sodium
phenoxide to form ether.
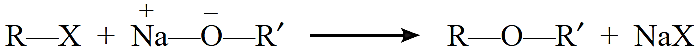
It is a convenient method for the preparation of symmetrical as well as unsymmetrical ethers.
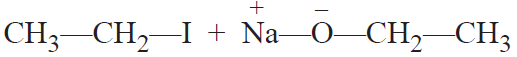
![]() CH3—CH2—O—CH2—CH3 + NaI
CH3—CH2—O—CH2—CH3 + NaI
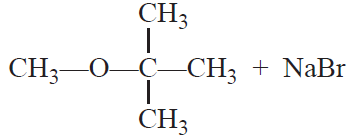
CH3—Br + 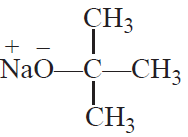
![]()
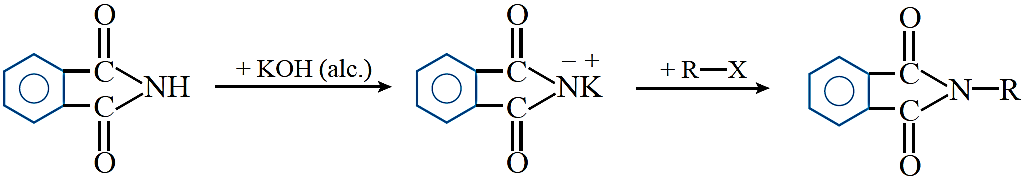
Gabriel phthalimide synthesis: This reaction is used for the preparation of aliphatic primary amines. In this reaction, phthalimide is first of all treated with ethanolic KOH to form potassium phthalimide. Potassium phthalimide on treatment with alkyl halide gives N-alkyl phthalimide, which on hydrolysis with dilute hydrochloric acid gives a primary amine as the product.
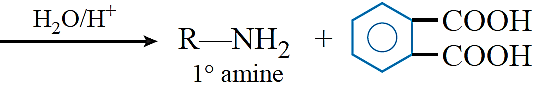
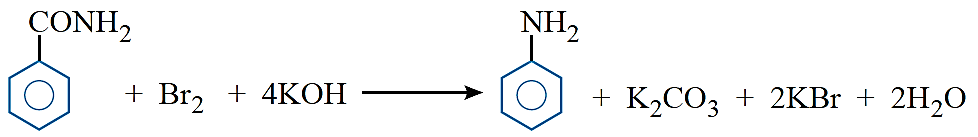
Hoffmann bromamide reaction: When a primary acid amide is heated with an aqueous or ethanolic solution of NaOH or KOH and bromine (i.e., NaOBr or KOBr), it gives a primary amine with one carbon atom less.
R—CONH2 + Br2 + 4NaOH → R—NH2 + Na2CO3 + 2NaBr +2H2O
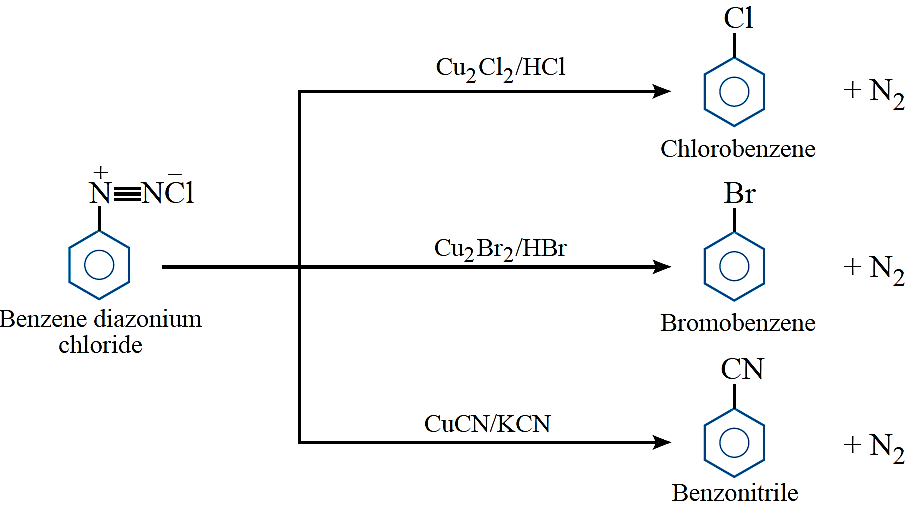
Sandmeyer’s reaction: The Cl–, Br– and CN – nucleophiles can easily be introduced in the benzene in the presence of Cu (I) ion. This reaction is called Sandmeyer’s reaction.
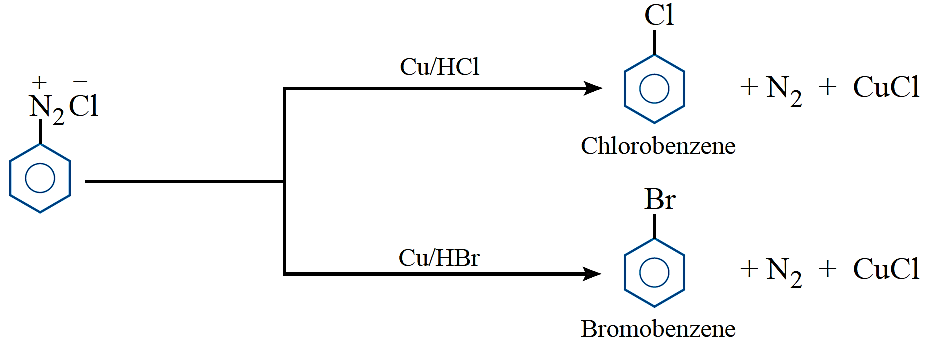
Gatterman’s reaction: Chlorine or bromine can be introduced in benzene ring by treating the diazonium salt solution with corresponding halogen acid in the presence of copper powder.
Carbylamine reaction (Isocyanide test): Aliphatic and aromatic primary amines when heated with chloroform and alcoholic solution of KOH give isocyanides (carbylamines) which have extremely unpleasant smell.
R—NH2+ CHCl3 + 3KOH (alc.) ![]() R—NC +3KCl +3H2O
R—NC +3KCl +3H2O
Kolbe’s reaction: When sodium phenoxide is heated with CO2 at 400 K under a pressure of 4 –7 atm, the resulting product on acidification yields salicylic acid.
Reimer–Tiemann reaction: Treatment of phenol with chloroform in the presence of sodium hydroxide followed by hydrolysis of resulting product gives o-hydroxybenzaldehyde (salicylaldehyde) as a major product.
Williamson synthesis: It consists of reacting an alkyl halide with sodium alkoxide or sodium
phenoxide to form ether.
R—X + Na—O—R′ →R—O—R′ + NaX
It is a convenient method for the preparation of symmetrical as well as unsymmetrical ethers.
CH3—CH2—I + Na—O—CH2—CH3 → CH3—CH2—O—CH2—CH3 + NaI