CONTENT BASED EXPERIMENTS
EXPERIMENT No 1(i)
Object – To prepare colloidal solution (Hydrosol) of starch.
Essential Instruments and Chemicals – Beakers (250 ml and 50 ml), glass rod, funnel, filter paper, pestle and mortar, tripod stand, wire-gauze and burner, Soluble starch (500 mg) and distilled water.
Principle-Starch forms a lyophilic sol when water is used as the dispersion medium. he formation of sol is accelerated by heating starch and water at about 100°C. It is quit stable and is not affected by the presence of any electrolytic impurity.
Procedure-
1. Take 500 mg of starch in mortar and add few ml of distilled water.
2. Grind the starch to make a thin paste and transfer this paste in a 50 ml breaker.
3. Take about 100 ml distilled water in a 250 ml beaker and heat the beaker so that water starts boiling.
4. Pour the paste slowly with stirring into boiling water in the beaker.
5. Continue boiling for about 10 minutes and then allow the beaker to cool.
6. F1ter the contents of the beaker through a filter paper, fixed in a funnel. Label this filtrate as ‘Starch Sol.
Result – The colloidal solution of starch is prepared.
Precautions-
1. The apparatus used for preparing sol should be properly cleaned.
2. Distilled water should be used for preparing sols in water.
3. Starch should be converted into a fine paste before adding to boiling water.
4. Starch paste should be added in a thin stream to boiling water.
5. Constant stirring of the contents is necessary during the preparation of the sol.
EXPERIMENT No 1(ii)
Object-To prepare colloidal solution (or sol) of egg albumin.
Essential Instruments and Chemicals- An egg and distilled water, Beakers (250 ml and 50 ml), glass rod, funnel, filter paper, pestle and mortar, tripod stand, wire gauge and burner.
Principle- Egg albumin which is obtained from eggs forms lyophilic sol with cold water, The sol is quite stable and is not affected by the presence of traces of impurities.
Procedure- Break the outer shell of the egg by stirring it with a glass rod and collect its colourless liquid along with yellow yolk. Decant the colourless liquid into another beaker. This colourless liquid is known as egg albumin. v:
2. Prepare 100 ml of 5% (w/V) solution of sodium chloride in a 250 ml beaker. To this solution add egg albumin is small portions with constant stirring, this process should take 15-20 minutes.
3. Filter the contents of the beaker through a filter paper fixed in funnel, and collect the filtrate. Label this filtrate as Egg-Albumin Sol.
Result-The colloidal solution of egg albumin is prepared.
Precautions-
1. The apparatus used for preparing the sol should be absolutely clean.
2. Distilled water should be used for, preparing the sol.
3. Egg albumin sol is prepared at room temperature because in hot solution the precipitation of egg albumin takes place.
4. The yellow yolk should be separated completely from the egg albumin before using the later in the experiment.
5. Addition of egg albumin should be done very slowly and with constant stirring so as to disperse the colloidal particles well in solution.
EXPERIMENT No 1(iii)
Object-To prepare the colloidal solution of Arabic gum.
Essential Instruments and Chemicals – Stop watch, beaker (250 ml), glass rod, tri-pod stand, burner, funnel, burette, wire gauze etc. Arabic gum 3-4 gram and, distilled water.
Principle- Arabic gum is prepared lyophilic sol with hot water.
Procedure
1. Solid Arabic gum is converted to powder state.
2. We take 150 ml hot water is 250 ml beaker and add to it small quantity of Arabic gum powder.
3. The mixture is regularly stirred with glass rod. This process take about 15 minutes.
4. After 5 minutes continued stirring all Arabic gum powder has been added hot water.
5. The suspended impurities are separated by filtration through is obtained as Arabic gum sol.
6. The filtrate is obtained as Arabic gum sol.
Result – The colloidal solution of Arabic gum is prepared.
Precaution-
1. Small amount of Arabic gum powder in added slowly-slowly in hot water.
2. Distilled water in used to prepare for sol.
EXPERIMENT No, 1
Object-To prepare colloidal solution of ferric hydroxide [Fe(OH)3] sol.
Essential Instruments and Chemicals – 2% Solution of ferric chloride (prepared by dissolving 2 gm of pure FeCl3 in 100 ml distilled water) and distilled water, Conical flask (250 ml), beaker (250 ml ), a boiling tube, glass rod, funnel, round-bottom flask, iron stand with a clamp, wire-gauze, tripod stand, burner, burette or a dropper.
Principle – Ferric hydroxide forms a lyophobic sol. The substances such as metal hydroxides or sulphides which are insoluble and do not readily give colloidal solutions on treatment with water are called lyophobic colloids.
Ferric hydroxide sol is prepared by the hydrolysis of ferric chloride with boiling distilled water. The reaction that takes place can be represented as :
FeCl3(aq) + 3H2O(l) → Fe(OH)3↓ +3HCI (aq)
Ferric chloride Red sol
The hydrolysis reaction produces insoluble ferric hydroxide particles which undergo agglomeration to yield bigger particles of colloidal dimensions. These particles adsorb Fe3+ ions preferentially from the solution to give positive charge on the sol partials. Stability of the sol is due to the charge on the sol particles. Hydrochloric acid which is produced during hydrolysis destabilizes the sol and hence it must be removed from the sol by dialysis process otherwise sol will not be stable.
Procedure-
1.Takea 250 ml conical flask and clean it by steaming-out process as show in.
2. To this cleaned flask, add 100 ml of distilled water and heat it to boil by placing the flask on a wire gauze.
3. Add ferric chloride solution dropwise (by the use of burette or a dropper) to the boiling water.
4. Continue heating until deep red or brown solution of ferric hydroxide is obtained. Replace the water lost by evaporation during boiling at regular intervals.
5. Keep the contents of conical flask undisturbed for sometime at room temperature.
Label this solution as “Ferric Hydroxide Sol”.
Result – The colloidal solution of ferric hydroxide [Fe(OH)3] sol.
Precautions
1.Snce ferric hydroxide sol is affected by impurities, the apparatus required for the preparation of sol should be thoroughly cleaned by steaming-out process.
2. Add ferric chloride solution dropwise.
3. Heating should continued till the desired sol is obtained.
4. Hydrochloric acid formed as a result of hydrolysis of ferric chloride is removed by dialysis process otherwise it would destabilize the sol.
Experiment No. 1(v)
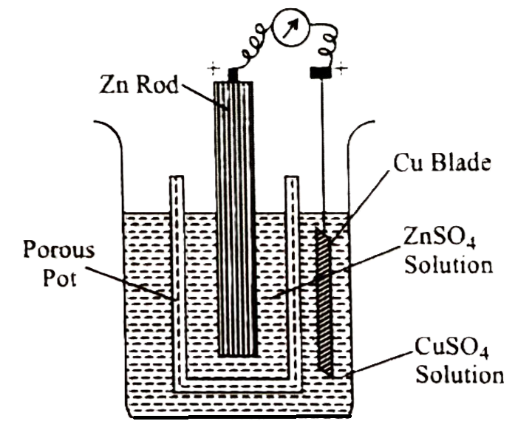
Object -To set up simple Daniell cell and determine its EMF.
Essential Instruments and Chemicals – One beaker (500 ml), a porous pot, connecting wires, milli voltmeter, sand paper, zinc strip, copper strip, 1M ZnSO4 solution and 1M CuSO4 solution.
Principle- When a copper electrode dipped in copper sulphate solution is connected to a zine electrode dipped in the zinc sulphate solution, then electrons fow from zine electrode to copper electrode and the chemical reactions take place as:
At Anode : Zn(s) →Zn2+(aq) + 2e–
At Cathode: Cu2+(aq) + 2e– → Cu(s)
Overall reaction : Zn(s) + Cu2+(aq) → Zn2+(aq) + Cu(s)
Procedure- 1. Take 1M CuSO4 solution in 500 ml beaker.
2. Clean the copper strip with the help of sand paper and dip it into 1M CuSO4 solution.
3. Take 1M ZnSO4 solution in a porous pot.
Daniell (Cell
4. Clean the zinc strip with the help of sand paper and it into zinc sulphate solution.
5. Keep the porous pot in the beaker.
6. Connect the copper strip with the positive terminal and zinc strip with the negative terminal of a voltmeter as shown in Figure.
7. Note the position of the pointer in the voltmeter and record the observations.
Result- EMF value of Daniell cell is 1.10 volt and find the value of EMF Daniell cell …. volt by experiment.
Precautions
1. Zn and Cu strip should by cleaned by sand paper.
2. The reading of the voltmeter should be taken after shaking the solution.
3. Connection should be the Cu strip. with the +ve terminal and Zn strip with -ve terminal.
EXPERIMENT NO. 2
Object- To purification of prepared sol by dialysis.
Essential Instruments and Chemicals- Parchment or cellophane paper-1 sheet, utensil, thread, test tubes, Sol solution, distilled water, AgNO3 solution, urenil zinc acetate solution.
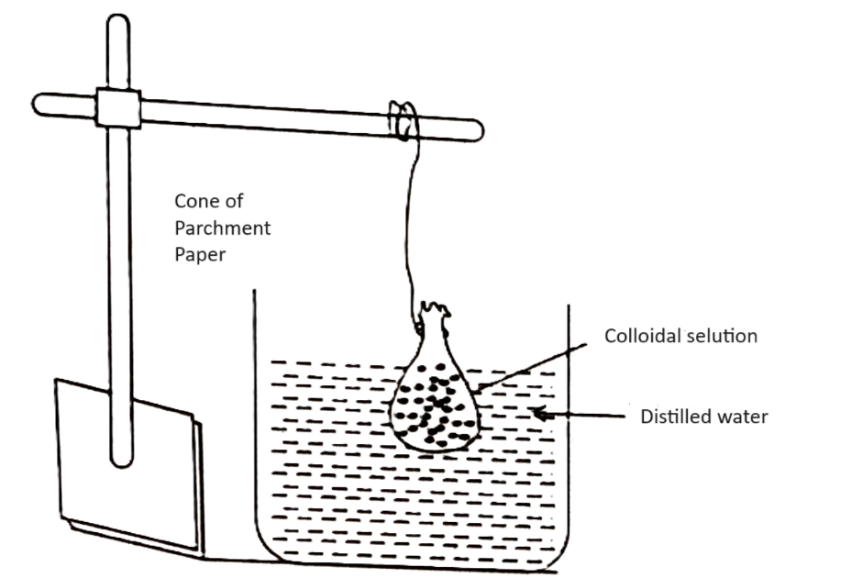
Principle- The ions of impurities comes out from parchment paper and remove aways with water colloidal particals can not comes out due to big size, thus the colloid becomes pure.
Diagram-
Purification of colloid by dialysis
Procedure
1. Fold the wet parchment paper of size 30 cm × 30 cm in a cone shape.
2. Fill the colloidal solution in it and now. tied the cone with a thread and hang it in a utensil filled with distilled water.
3. Now, observe the presence of ions in water after half an hour. Now change the water of utensil after half an hour repeatedly.
4. To identify the ionic impurities Na+ and Cl–, take the water of utensil in two test tubes, Add urenil zinc acetate solution in one test tube, yellow precipitate show the presence of Na+ ions. Add silver nitrate solution in second test tube, white ppt. show the presence of Cl– ions.
5. Note the time for purification of colloidal particles.
6. When yellow or white ppt stops to come out, then pure collide is obtained.
Result-The pure colloidal solution of sol is obtained by dialysis method.
Precautions-
1. The cone of parchment paper tied by thread strongly, Water should not go inside the cone.
2. The neck of the cone remains up from the surface of water such that.
3. The water of utensil change time to time.
EXPERIMENT No 3
Object-To study the effect of temperature on the reaction between sodium thiosulphate and hydrochloric acid.
Essential Instruments and Chemicals – Pipette 10 ml, 150 ml conical -flask, thermometer, wire gauze, tripod stand, stopwatch and white tile with a cross mark, 0.1 M Na2S2O3 solution 1M HCl, conc. HNO3 and distilled water.
Principle – The rate of a chemical reaction generally depends on the temperature. The rate of reaction increases with increase in temperature. The rate is doubled by a 10°C increase in temperature.
Procedure-
1. Take 50 ml 0.1 M Na2S2O3 solution in a conical flask and measure its temperature, Let it be 30°C.
2. Add 5 ml 1M HCI in the conical Alask and immediately start the stop watch.
3. The cross mark is viewed from the top and the time when the mark is not clearly visible is noted.
4. Repeat the same procedure at temperature 40°C, 50°C, 60°C and 70°C. Note the time when the cross mark becomes invisible.
Vourne of Na2S2O3 in every measurement = 50 ml
Volume of 1M HCl added = 5 ml
|
S.No. |
Concentration of Na2SO3 Solution |
Time taken when the cross mark is invisible |
|
1 |
0.1 |
t1 = 12 sec |
|
2 |
0.2 |
t2 = 9 sec |
|
3 |
0.3 |
t3 = 7 sec |
|
4 |
0.4 |
t4 = 5 sec |
|
5 |
0.5 |
t5 = 3 sec |
Plotting Graph
A graph is plotted between temperature on the X-axis and time on the Y-axis.
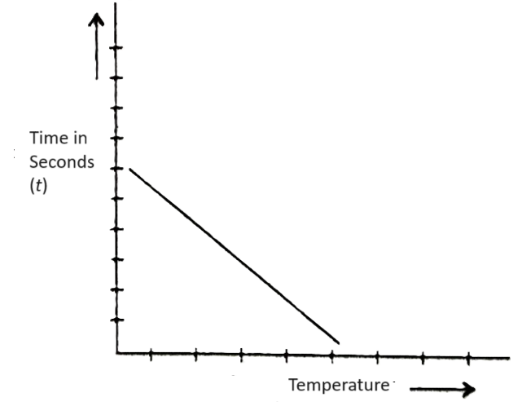
Result – The rate of reaction between Na2S2O3 and HCl increases with increase in temperature.
Precaution- 1. The apparatus should be clean.
2. The same tile with a cross mark should be used in every experiment.
3. The stop watch should be started immediately upon the addition of HCl to Na2S2O3 solution.
4. The cross mark should be viewed from the top in every measurement.
Experiment
Object – To study the rate of reaction between potassium iodate and sodium sulphite using starch as indicator.
Essential Instruments and Chemical – Six conical flask of capacity 250 ml, measuring cylinder (100mL), stop watch, 0.01 M KIO solution (potassium iodate), 0.005 M sodium sulphide, sulfuric acid, stop watch, potassium iodate, sodium sulphite, 2M H2SO4, starch, distilled water etc.
Principle- Iodine is formed by the reaction of iodide ions in potassium iodate and sodium sulphate.
IO3– + 3SO32- → I–+3SO42- ………………(i)
5I– +6H+ +IO3– → 3H2O +3I2 ……………(ii)
This reaction is also a clock reaction
Procedure
(i) Take five 250, 250ml conical flasks and mark them as A, B, C, D, E.
(ii) 2 mL of 0.01 M potassium iodate solution is measured in a measuring cylinder in conical flask A, 4 mL in B, 6 mL in C, 8 mL in D and 10 mL.
(iii) From the measuring cylinder, 10 ml solution of M sulfuric acid is added to each of A, B, C, D, E chronic flasks.
(iv) Now, measuring with a measuring cylinder, add so much distilled water to each conical flask that the total volume of the solution in the conical flask becomes 100 mL, that is, 88 mL in conical flask A. distilled water, in B 86ml, 84ml in C and 82mL in D, 80ml of distilled water is added to E.
(v) To each conical flask add 10 ml of 0.005 M solution of freshly prepared starch solution.
(vi) Now take 10 ml of 0.005 M solution of sodium sulphide in conical flask A and immediately start the stop watch, stop the watch as so0n as blue colour appears and note the time taken for blue colour to appear.
(vii) Follow the same procedure for conical flask B, C, D, E and note the time of appearance of blue colour.
(viii) Record the observations in the observation table.
|
Name of Flask |
0.01 M KIO3 Solution (mL) |
1/KIO3 Solution |
1 M H2SO4 Solution |
Water (mL) |
Starch Solution (mL) |
0.005 M Na2SO3 Solution |
Time of appearance blue colour (Sec.) |
|
A |
2 |
0.5 |
10 |
88 |
5 |
10 |
75 |
|
B |
4 |
0.25 |
10 |
86 |
5 |
10 |
35 |
|
C |
6 |
0.167 |
10 |
84 |
5 |
10 |
23 |
|
D |
8 |
0.125 |
10 |
82 |
5 |
10 |
14 |
|
E |
10 |
0.1 |
10 |
80 |
5 |
10 |
10 |
Result- On drawing the graph between 1/KIO3, solution and the time taken for blue colour to appear, a straight line is obtained from which it can be concluded that the rate of reaction between potassium iodate and sodium sulphite is Increases with increasing concentration of iodate.
Precautions
(i) The concentration of potassium iodate should be more than that of sodium sulphite.
(ii) Freshly prepared starch solution is used.
(iii) Freshly prepared sodium sulphite solution is used.
(iv) Note the time as soon as blue colour appears.
Object- Calculate the enthalpy change in the formation of aquous solution of CuSO4(s) at room temperature.
Essential Instruments and Chemicals – Beaker (250 ml) Rubber cork with two holes, thermometer (till 0.5°O), agitator, measuring cylinder, CuSO4.5H0 powder, distilled water.
Principle- The value of calorimeter constant is given by the formula—
![]()
Solvation Enthalpy: It is heat release or absorbed in the process if one mole solute is dissolved in sufficient quantity of water so the heat change on dilution is negligible. The value of solvation enthalpy.
ΔH = ![]()
Here, x = amount of CuSO4, W = Calorimeter constant
t4 = Initial temp. of distilled water
t5 = Temp. of solution after CuSO4 dissolved
Procedure-
(A) To calculate calorimeter constant :
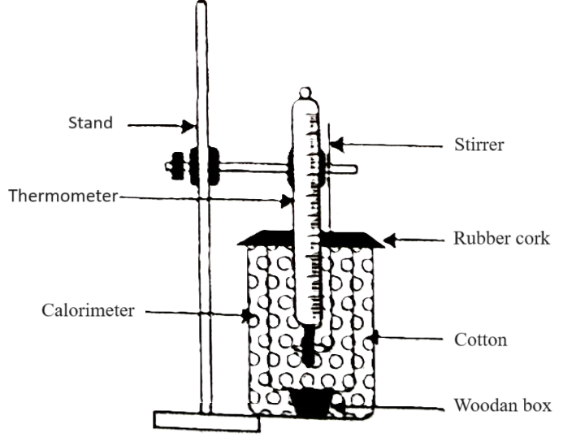
1. Take a 250 ml beaker, put thermometer and stirrer in two different hole cork.
2. Take 100 ml distilled water at normal temperature creature by measuring cylinder. Put the cork with thermometer and stirrer on it.
3. Take a heat resistant box and place the beaker in it and note the temp (t1°C)
4. Take 100 ml hot water (50 – 60°C) in another beaker and note the temp t1°C).
5. Now mix this hot water in the beaker placed which is placed in heat resistant box. After mixing stir the water by stirrer and not the temp. of mixture (t3°C).
Determination of Colorimeter Constant
(B) To calculate the enthalpy change in solution :
1. Take 100 ml distilled water of known calorimeter constant and placed it in a heat resistant box. Now note the temp (t4°C).
2. A certain let x gram amount of powdered CuSO4 added in the water of calorimeter.
3. Stir well by stirrer till complete dissolvation.
4. Note the temperature of CuSO4 solution (t5°C).
5. Volume of normal water = 100 ml and temp = t1°C
Volume of hot water 100 ml and temp t2°C
Temperature of mixture = t3°C
Quantity of CuSO4 used = x gm
Volume of water before addition of CuSO4 = 100 ml and temp. t4°C.
Temperature of CusO4 solution = t5°C
Calculation-
Enthalpy change can be calculate by the following relation.
Total mass of solution = (100 + x) gm
Temperature change = Δt = t4°C – t5°C
Energy absorbed by solution = (100 + x) (t5 -t4) 4.2 J
Calorimeter constant = W and Temperature change = Δt= t5– t4
Energy absorbed by calorimeter = W (t5 – t4) 4.2 J
Total enthalpy change = 4.184 (100 + x) (t5 – t4) + W (t5 – t4)
This enthalpy change occurs due to dissolution of x gm CuSO4. So enthalpy change due to dissolution of 1 mole (249.5 gm) CuSO4 is given by –
ΔH= ![]() Jmol-1
Jmol-1
Result – The value of dissolution enthalpy of CuSO4 is …………… Jmol-1
Precaution
1. All apparatus should be heat and cleaned.
2. All temperatures should be noted quickly.
EXPERIMENT No. 6
Object – To calculate the enthalpy change for neutralisation of a strong acid (HCl) by a strong base (NaOH).
Essential Instruments and Chemicals – Three beaker (250 ml), one beaker (500 ml), glass rod, rubber cork with two holes, thermometer, agitator, measuring cylinder, cotton, small ice of wood, card board piece, 1.0 M HCI solution, 1.0 M NaOH solution.
Principle- When acid and base are mixed, they neutralize to each other. In this reaction hydrogen ions of acid react with hydroxyle ions of base to form water
H+(aq) + OH– (aq) → H2O(l) : Δnest(H) =-QKJ
where Δnest(H) is the enthalpy of neutralisation. Neutralisation heat of strong acid and strong base is 57.32 kJ heat at 20°C.
Procedure-
(A) To determine the calorimeter constant: Do it as previously done.
(B) To determine the enthalpy of neutralisation,
1. Take 1.0M HCl solution in calorimeter and note the temperature.
2. Take 1.0M NaOH solution in other beaker and note the temperature.
3. Suppose both are at equal temp t1°C.
4. Mix the 100 ml NaOH solution in the HCl solution of calorimeter.
5. Cover it with rubber cork having thermometer and agitator.
6. Note the temperature when becomes constant suppose it is t2°C.
Calculation-
Temperature of both solutions = t1°C
Total volume of solution =100+ 100 = 200 ml
Final temperature of mixture = t2°C
Increase in temperature due to releasing heat = (t2 -t1)° C
Calorimeter constant of calorimeter = WJ/°C
Heat released due to neutralisation = Heat absorbed by mixture + Heat absorbed by calorimeter
= (200 + w) × (t2 -t1) × 4.184 J
The heat released due to mixing of 1000 ml of 1.0 M HCI and 1.0 M NaOH solution.
![]()
= ![]() kJ
kJ
Result
The enthalpy of neutralisation = ………….. kJ mol-1
Precautions-
1. The temperature should be noted carefully after the mixing of base in acid and stirring.
2. The Concentration and volume of strong acid and strong base should be same.
EPEREMENT No 7
Object– To determine the enthalpy change during the interaction (hydrogen bond formation) between acetone and chloroform.
Essential Instruments and Chemicals – A wide mouthed polythene bottle fitted with a thermometer (1/10th degree) and a stirrer (to serve as calorimeter), 100 ml measuring cylinder, Pure acetone and pure chloroform.
Principle – When acetone mixed with Chloroform, heat is evolved due to formation of hydrogen bonds between chloroform and acetone:
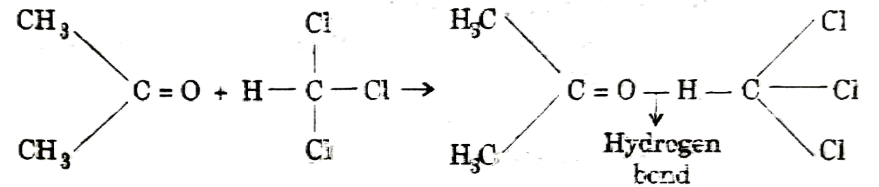
Heat evolved during this interaction can be experimentally by mixing the liquids and measuring the heat change by suing a calorimeter.
Procedure –
To determine of calorimeter constant
1. Put 100 ml of distilled water in polythene bottle with a thermometer and stirrer Fig.
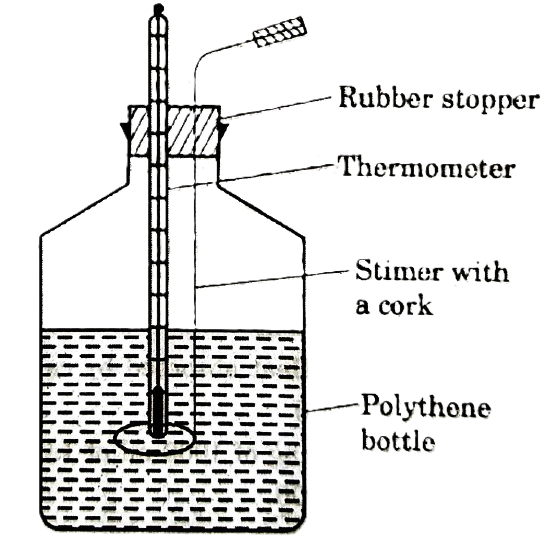
Polythene bottle calorimeter
1. Note the temperature (t1°C).
2. Heat some water in a beaker to a temperature 20-30°C higher than that of room temperature.
3. Put 100 ml of this hot water in another beaker.
4 Note the temperature of this water, Let it be t2°C.
5. Add hot water from the beaker into the polythene bottle.
6. Read the temperature attained after mixing. Let it be t3°C.
(B) To determination of enthalpy of interaction of acetone and chloroform
1. Take a polythene bottle calorimeter and place 100 ml acetone in it. Note the temperature of acetone,
2. Take 100 ml of chloroform in a beaker and note its temperature. Both the solutions should have same temperature otherwise walt for some time so that they attain same temperature,
3. Transfer the chloroform into the calorimeter and immediately ft the cork having thermometer and stirrer. Stir gently.
4. Note the temperature after small intervals till it becomes constant. Record the highest temperature reached.
Calculation-
Initial temperature of acetone and chloroform = t1°C
Final temperature after mixing the two liquids = t2°C
Change in temperature = Δt (t1 – t1)°C
Calorimeter constant of calorimeter =W J/°C
Density of Chloroform = 1.499 g/cm3
Density of acetone = 0.787 g/cm3
Heat capacity of chloroform, S1 = 0.96 J/g
Heat capacity of acetone, S = 2.18 J/g
Heat change = W × 4.184 x (t2 – t1) + [100 × 1.499 × S1 + 100 × 0.787 × S] (t2 – t1)
Joules = …………….. Joules
Since t2 > t1 in this experiment, heat is evolved and enthalpy change for the interaction of acetone and chloroform has negative sign.
Result – Enthalpy change during mixing of 100 ml of acetone with 100 ml of chloroform = ……….. Joules.
