NCERT CHEMISTRY FOR 11
CHAPTER STRUCTURE OF ATOM
Q. 1 Rutherford’s scattering experiment is related to the size of the
(a) Nucleus (b) atom (C) neutron (d) proton
Q. 2 Lines in the hydrogen spectrum which appears in the infrared region of the electromagnetic Spectrum, are called as
(a) Balmer series (b) Hydrogen line series
(c) Hydrogen series (d) Paschen series
Q.3 Which of the following statement is not correct about the characteristics of cathode rays?
(a) Characteristics of cathode rays do not depend upon the material of electrodes in cathode ray tube.
(b) They start from the cathode and move towards the anode.
(c) They travel in straight line in the absence of an external electrical or magnetic field.
(d) Characteristics of cathode rays depend upon nature of gas present in the cathode ray tube.
Q. 4 Be2+ is isoelectronic with which of the following ions?
(a) H+ (b) Li+ (c) Na+ (d) Mg2+
Q. 5 Which of the following is the correct electronic configuration of Fe2+ ion (Z for Fe = 26)?
(a) 1s2 2s 2 2p6 3s2 3p6 3d 4 4s2
(b) 1s 2 2s2 2p6 3s2 3P6 3d6
(c) 1s2 2s2 2p6 3s2 3p6 4s2 4P4
(d)1s2 2s2 2p6 3s2 3P6 3d5 4s1
Q. 6 p-orbital can accommodate
(a) 4 electrons
(b) 6 electrons
(c) 2 electron with Parallel spins
(d) 2 electrons with opposite spins
Q. 7 The principal quantum number of an atom is related to the
(a) size of the orbital
(b) spin angular momentum
(c) orbital angular momentum
(d) orientation of the orbital in Space
Q. 8 According to the Bohr theory, which of the following transitions in the hydrogen atom will give rise to the least energetic photon?
(a) n = 6 to n = 1
(b) n = 5 to n = 4
(c) n = 6 to n = 5
(d) n = 5 to n = 3
Q. 9 Which one is a wrong statement?
(a) Total orbital angular momentum of electron in s-orbital is equal to zero.
(b) An orbital is designated by three quantum numbers while an electron in an atom is designated by four quantum numbers.
(c) The electronic configuration of N atom is 1s2 2s2 2px1 2py2 2pz0
(d) The value of m for dz2 is zero.
Q. 10 Orbital having 3 angular nodes and 3 total nodes is
(a) 5p (b) 3d (c) 4f (d) 6d
Q. 11 Which of the following statement does not form a part of Bohr’s model of hydrogen atom?
(a) Energy of the electron in the orbit is quantized
(b) The electron in the orbit nearest to the nucleus has the lower energy
(c) Angular momentum of the electron in the orbit is quantized
(d) The position and velocity of the electrons in the orbit cannot be determined simultaneously.
Q. 12 Two electrons occupying the same orbital are distinguished by
(a) Azimuthal quantum number
(b) spin quantum number
(c) principal quantum number
(d) magnetic quantum number.
Q. 13 The maximum number of electrons in a sub shell is given by the expression
(a) 4l – 2 (b) 4l + 2 (c) 2l + 2 (d) 2n2
Q. 14 Arrange the following particles in increasing order of values of elm ratio :
electron (e), proton (p), neutron (n), alpha particle (α)
(a) n, p, e, α (b) n, α., p, e (c) n,p, α ,e (d) e, p, n, α.
Q. 15 The shortest wavelength in H spectrum of Lyman series when RH = 109678 cm-1 is –
(a) 1215.67 A (b) 911.7 A (c) 1002.1 A (d) 1127.30 A
Q.16 Naturally occurring boron consists of two isotops whose atomic weights are 10.01 and 11.01. The atomic weight of natural boron is I 0.81. Calculate the
percentage of each isotope in natural boron-
(a) 20,80 (b) 30,70 (c) 10,90 (d) 15,85
Q. 17 The wave-mechanical model of atom does not support
(a) De Broglie concept of dual character of matter
(b) Heisenberg’s uncertainty principle
(c) Bohr’s model of H atom
(d) Schrodinger wave equation
Q. 18 The orbital having m = – 2 should not be present in the following sub-shell
(a) d (b) f (c) g (d) p
Q. 19 Choose the permitted quantum numbers among the following sets –
(a) n = 3, l=2,m=- 1, s=O
(b) n = 2., l=2, m=+l, s= -1/2
(c) n=2, l=2, m=+ 1, s = +1/2
(d) n=3, l=2. m=-2, s= +1/2
Q. 20 Choose the wrong statement-
(a) Shapes of orbitals are functional representation of mathematical solutions of Schrodinger equations. They do not represent any picture of electric charge on matter.
(b) Smaller the wavelength of the electron wave more is the resolving power of the electron microscope.
(c) Uncertainty in measurement is not due to lack of any experimental technique but due to nature of subatomic particle itself.
(d) The K.E. of electrons ejected from the metal surface is proportional to the intensity or brightness of light.
Q. 21 In hydrogen atom, energy of first excited state is –3.4 eV. Then find out K.E. of same orbit of hydrogen atom.
(a) +3.4 eV (b) +6.8 eV (c) –13.6 eV (d) +13.6 eV
Q. 22 In a given atom no two electrons can have the same values for all the four quantum numbers. This is called
(a) Hund’s Rule
(b) Aufbau principle
(c) Uncertainty principle
(d) Pauli’s Exclusion principle.
Q. 23 Calculate the wavelength of a moving electron having 4.55 x 10-25 J of kinetic energy-
(a) 6.27 x10– 7 meter
(b) 7.27 x 10– 6 meter
(c) 6.27 x 10 -7 meter
(d) 12.27 x 10 -7 meter
Q. 24 The probability density plots of 1s and 2s orbitals are given in following Fig. the density of dots in a region represents the probability density of finding electrons in the region. On the basis of diagram given below which of the following statements is incorrect?
(a) 1s and 2s orbitals are spherical in shape.
(b) The probability of finding the electron is maximum near the nucleus.
(c)) The probability of finding the electron at a given distance is equal in all directions.
(d) The probability density of electrons for 2s orbital decreases uniformly as distance from nucleus increases
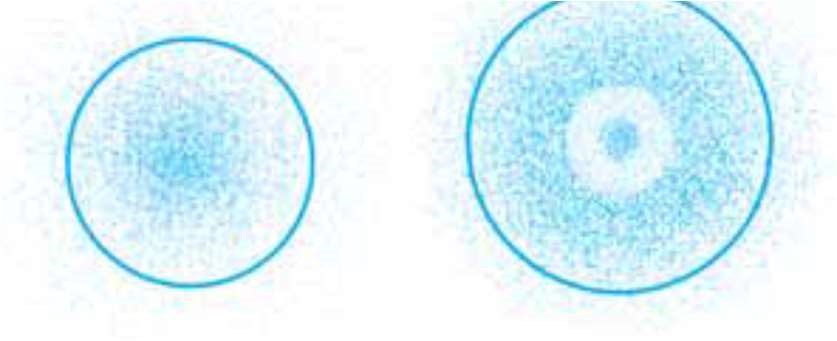
.
Q. 25 According to quantum mechanics ψ2(r) the wave function squared gives:
(a) probability of finding a neutron
(b) probability density of finding an electron
(c) probability of finding an electron finding a proton
(d) probability density of
Q. 26 Emission spectrum of a material results from the material’s (atom ion or molecules)
(a)Transition from normal to excited state
(b) Radiating a continuous spectrum
(c) transition from excited to normal state
(d) Radiating an inverted spectrum
Q. 27 Suppose atomic radius of an atom is of the order of 10-8 cm. and nuclear radius is of the order of 10-13 cm. Calculate what fraction of atom is occupied by nucleus?
(a) 10-10 (b) 10-15 (c) 10-12 (b) 10-9
Q. 28 The orbital angular momentum of a p-electron is given as
(a) h / ∫2 π (b) ∫3 h/ 2π (c) ∫ 3/2 h/p (d) ∫ 6 h/2 π
Q. 29 A source of monochromatic radiation of wavelength 400 nm provides 1000 J of energy in 10 seconds. When this radiation falls on the surface of sodium, x × 1020 electrons are ejected per second. Assume that wavelength 400 nm is sufficient for ejection of electron from the surface of sodium metal. The value of x is ________ .
(Nearest integer) (h = 6.626 × 10–34 Js)
( a) 2 (b) 3 (c) 1 (d) 4
Q. 30 The radius of which of the following orbit is same as that of the first Bohr’s orbit of H atom
(a) He+ (b) He2+ (c) Li 3+ (d) Be 3+
Q. 31 The wavelength of electrons accelerated from rest through a potential difference of 40 kV is X × 10–12 m. The value of X is _______. (Nearest integer)
Given : Mass of electron = 9.1 × 10–31 kg
Charge on an electron = 1.6 × 10–19 C
Planck’s constant = 6.63 × 10–34 Js
Q. 32 The study of emission or absorption spectra is referred to as ————-
Q. 33 The splitting of spectral lines in the presence of magnetic field is called as —————- or an electric field —————–
Q. 34 The effect of Heisenberg Uncertainty Principle is significant only for motion of —————-objects and is negligible for that of macroscopic objects
Q. 35 An atomic orbital is the ——————-for an electron in an atom.
Q. 36 The region where this probability density function reduces to zero is called ——————-or simply nodes
Q. 37 The orbitals having the same energy are called———————————–.
Q. 38 The maximum number of atomic orbitals associated with a principal quantum number 5 is ——————————
Q. 39 In 1830, ——————showed that if electricity is passed through a solution of an electrolyte, chemical reactions occurred at the electrodes.
Q. 40 Bohr’s atomic theory is not able to explain the atomic spectra of atoms containing ________ electron.
In the following questions a statement of Assertion (A) followed by a statement of
Reason (R) is given. Choose the correct option out of the choices given below each question
Q. 41 Assertion (A) : : The energy of an electron is largely determined by its principal quantum number.
Reason(R) : The principal quantum number is a measure of the most probable distance of finding the electrons around the nucleus.
(a) Both A and R are true and R is the correct explanation of A.
(b) Both A and R are true but R is not the correct explanation of A.
(c) A is true but R is false.
(d) A is false bur R is true
(e) Both A and R are false
Q. 42 Assertion (A): The opposite lobes of a p-orbital have opposite sign whereas opposite lobes of d-orbital have the same sign.
Reason (R) : The opposite lobes of a p-orbital have opposite charge whereas the opposite lobes of d-orbital have the same charge.
(a) Both A and R are true and R is the correct explanation of A.
(b) Both A and R are true but R is not the correct explanation of A.
(c) A is true but R is false
(d)A is false bur R is true
(e) Both A and R are false
Q. 43 Assertion (A) : Wave number of a spectral line for an electronic transition is quantised.
Reason (R) : It is proportional to the velocity of the electron undergoing the transition.
(a) Both A and R are true and R is the correct explanation of A.
(b) Both A and R are true but R is not the correct explanation of A.
(c) A is true but R is false
(d)A is false bur R is true
(e) Both A and R are false
Q. 44 Assertion (A) : It is impossible to determine the exact position and exact momentum of an electron simultaneously.
Reason (R) : The trajectory of electron in an atom is clearly defined.
(a) Both A and R are true and R is the correct explanation of A.
(b) Both A and R are true but R is not the correct explanation of A.
(c) A is true but R is false
(d)A is false bur R is true
(e) Both A and R are false
Q. 45 Assertion (A) : p-orbital is dumb-bell shaped
Reason (R) : Electrons present in P-orbital can have any one of the three values of magnetic quantum number t. e. + 1,0, -l
(a) Both A and R are true and R is the correct explanation of A.
(b) Both A and R are true but R is not the correct explanation of A.
(c) A is true but R is false.
(d)A is false bur R is true
(e) Both A and R are false
Q. 46 Assertion (A) : The 19th electron enters into the 4s orbital not in 3d orbital.
Reason (R) : (n+l) rule is followed for determinig the orbital of the lower energy.
(a) Both A and R are true and R is the correct explanation of A.
(b) Both A and R are true but R is not the correct explanation of A.
(c) A is true but R is false.
(d)A is false bur R is true
(e) Both A and R are false
Q. 47 Assertion (A) : Black body is an ideal body that emits and absorbs radiations of all frequencies.
Reason (R) : The frequency of radiation emitted by a body goes from a lower frequency to higher frequency with an increase in temperature,
(a) Both A and R are true and R is the correct explanation of A.
(b) Both A and R are true but R is not the correct explanation of A.
(c) A is true but R is false.
(d) A is false bur R is true
(e) Both A and R are false
Q. 48 Assertion (A) : The radius of the first orbit of hydrogen atom is 0.529Å.
Reason (R): Radius of each circular orbit (rn) – 0.529Å (n2/Z), where n = 1, 2, 3 and Z = atomic number.
(a) Both A and R are true and R is the correct explanation of A.
(b) Both A and R are true but R is not the correct explanation of A.
(c) A is true but R is false.
(d) A is false bur R is true
(e) Both A and R are false
Q. 49 Assertion (A): A few positively charged ∝–particles in alpha rays experiment were deflected by small angles.
Reason (R) : The radius of the atom is about 10–10 m, while that of nucleus is 10–15 m.
(a) Both A and R are true and R is the correct explanation of A.
(b) Both A and R are true but R is not the correct explanation of A.
(c) A is true but R is false.
(d) A is false bur R is true
(e) Both A and R are false
Q. 50 Assertion (A) : It has been observed that though the number of electrons ejected in a photocell does depend upon the brightness of light, the kinetic energy of the ejected electrons does not. K. E. depends upon the frequency of light (E= hn)
Reason (R) : Whenever radiation interacts with matter, it displays particle like properties in contrast to the wavelike properties (interference and diffraction), which it exhibits when it propagates. This concept
(a) Both A and R are true and R is the correct explanation of A.
(b) Both A and R are true but R is not the correct explanation of A.
(c) A is true but R is false.
(d) A is false bur R is true
(e) Both A and R are false
Q. 51 Define
(a) photoelectric effect (b) isotope
Q. 52 (a) Write the electronic configuration of Fe2+ ion.
(b) How many unpaired electrons are present in Pd (Z = 46)?
Q. 53 What is the difference between the terms orbit and orbital?
Q. 54 A hypothetical electromagnetic wave is shown in the figure given below . Find out the wavelength and the frequency of the radiation.
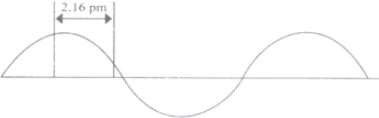
Q. 55 Calculate the mass and charge of one mole of electrons.
Q. 56 State Pauli exclusion principle and its consequences in the electronic configuration.
Q. 57 The unpaired electrons in Al and Si are present in 3p orbital. Which electrons were experience more effective nuclear charge from the nucleus? And why ?
Q. 58 (a) How many sub shells are associated with n = 4?
(b) How many electrons will be present in the sub shell having ms value of – 1 /2 for n=4?
Q. 59 Which principle of quantum mechanical model of the atom is responsible to rule out the existence of definite paths or trajectories of electrons? And how?
Q. 60 In Rutherford’s experiment, generally the thin foil of heavy atoms, like gold, platinum etc. have been used to be bombarded by the α -particles. If the thin foil of light atoms like aluminium etc. is used, what difference would be observed from the above results?
Q. 61 Calculate the uncertainty in the momentum of an electron if it is confined to a linear region of length 1 × 10 -10
Q. 62 (a) The diameter of zinc atom is 2.6A0 . Calculate
(i). the radius of zinc atom in pm
(ii). number of atoms present in a length of 1.6 cm if the zinc atoms are arranged side by side lengthwise.
(b) 2 ×108 atoms of carbon are arranged side by side. Calculate the radius of carbon atom if length of this arrangement is 2.4 cm.
Q. 63 What is the main difference between electromagnetic wave theory and Plank’s quantum theory ?
Q. 64 Which of the following relate to wave nature of light or particle nature or both?
(a) Interference
(b) Black body radiation
(c) Planck,s equation E = hv
(d) Einstein equation E = mc2
Q. 65 What did Rutherford conclude from the observations of α -ray scattering experiment?
Q. 66 What is the energy in joules, required to shift the electron of the hydrogen atom from the first Bohr orbit to the fifth Bohr orbit and what is the wavelength of the light emitted when the electron returns to the ground state?
Q. 67 With what velocity must an electron travel so that its momentum is equal to that of a photon of wavelength λ = 5200 A 0 ?
Q. 68 Chlorophyll present in green leaves of plants absorbs light at 4.620 × 1014 Hz.
Calculate the wavelength in nanometre.
Which part of the electromagnetic spectrum does it belong to?
Q. 69 The electronic configuration of valence shell of Cu is 3d104s1 and not 3d94s2. How is this configuration explained?
Q. 70 Name the elements in each of the following cases :
(i) A bivalent anion of the element having 10 electrons
(ii) A trivalent cation of the element having 10 electrons
What is the relationship between the two ions called?
Q. 71 According to de Broglie, matter should exhibit dual behaviour that is both particle and wave like properties. However, a cricket ball of mass 100 g does not move like a wave when it is thrown by a bowler at a speed of 100 km/h. Calculate the wavelength of the ball and explain why it does not show wave nature?
Q. 72 What were the observations which lead the J.J.THOMSON to conclude that –
(a)Cathode rays travel in the straight line
(b) cathode rays are made up of negatively charged particles
(c) cathode rays are particle waves.
Q. 73 Account for the following –
(a) cathode rays are produced only when the Pressure of the gas in the discharge tube is very low.
(b) Bohr’s orbits are called stationary orbits.
(c) we don’t draw a boundary surface diagram within which the probability of finding the electron is 100%?
Q. 74 Which experiment led to the discovery of electrons and how?
Q. 75 An ion with mass number 37 possesses one unit of negative charge. If the ion contains 11.1% more neutrons than the electrons, find the symbol of the ion.
Q. 76 What is the experimental evidence in support of the idea that electronic energies in an atom are quantized?
Q. 77 (a) In Rutherford’s experiment, generally the thin foil of heavy atoms, like gold, platinum etc. have been used to be bombarded by the -particles. If the thin foil of light atoms like aluminium etc. is used, what difference would be observed from the above results?
(b) Find the size of the chart paper used to draw an atom in which 1 rupee coin (diameter is app. 20mm) is used to draw the nucleus of that atom .
Q. 78 Answer the following questions –
(a) Nickel atom can lose two electrons to form Ni2+ ion. The atomic number of nickel is 28. From which orbital, will nickel lose two electrons?
(b) Which of the following orbitals are degenerate: 3dxy, 4dxy, 3dz2, 3dyz,4dyz,4dz2
(c) Which of the following will not show deflection from the path on passing through an electric field?
Proton, cathode rays, electron, neutron.
Q. 79 Lifetimes of the molecules in the excited states are often measured by using pulsed radiation source of duration nearly in the nano second range. If the radiation source has the duration of 2 ns and the number of photons emitted during the pulse source is 2.5X 1015, Calculate the energy of the source.
Q. 80 What were the weaknesses or limitations of Bohr’s model of atoms? Briefly describe the quantum mechanical model of atom?
Q. 81 Two particles X and Y in motion. If the wavelength associated with particle X is 4 x 10-4 nm, calculate the wavelength associated with particle Y if its momentum is half of X.
Q. 82 If uncertainty in position of an electron is same as the Δx of He atom. If Δp of e is
3.2x 105 Kg ms-1 find Δp in the He atom.
Q. 83 What is photoelectric effect? State the result of photoelectric effect experiment that could not be explained on the basis of laws of classical physics. Explain this effect on the basis of quantum theory of electromagnetic radiations.
Q. 84 What are the two longest wavelength lines (in nanometres) in the Lyman series of hydrogen spectrum?
Q. 85 The ejection of the photoelectrons from the silver metal in the photoelectric effect experiment can be stopped by applying the voltage of 0.35 V when the radiation 256.7 nm is used. Calculate the work function for silver metal.
Q. 86 Read the passage given below and answer the following questions:
In view of the shortcoming of Bohr’s model, attempts were made to develop a more suitable and general model for atoms. Two important developments which contributed significantly in the formulation of such a model were the Dual behaviour of matter, Heisenberg uncertainty principle. Uncertainty principle which is the consequence of the dual behaviour of matter and radiation. It states that it is impossible to determine simultaneously, the exact position and exact momentum (or velocity) of an electron. If the position of the electron is known with a high degree of accuracy ( ∆x is small), then the velocity of the electron will be uncertain (∆v) is large]. Physical measurements on the electron’s position or velocity, the outcome will always depict a fuzzy or blur picture. One of the important implications of the Heisenberg Uncertainty Principle is that it rules out the existence of definite paths or trajectories of electrons and other similar particles.
(a) Show that the circumference of the Bohr orbit for the hydrogen atom is an integral multiple of the de Broglie wavelength associated with the electron revolving around the orbit.
(b) What are the limitations of Heisenberg’s Uncertainty Principle?
(c) If the uncertainty in the velocities of two particles A and B with masses of 1.0 × 10-27 kg and 1.0 × 10-31 kg, respectively, is the same, what will be the ratio of uncertainty in their positions?
(d) What is the order of wavelength associated with a 200g golf ball moving at a speed of 5mh–1?
Q. 87 Read the passage given below and answer the following questions:
A large number of orbitals are possible in an atom. Qualitatively these orbitals can be distinguished by their size, shape and orientation. An orbital of smaller size means there is more chance of finding the electron near the 5 / 10 nucleus. Similarly, shape and orientation mean that there is more probability of finding the electron along with certain directions than along others. The principal quantum number determines the size and to large extent the energy of the orbital. Azimuthal quantum number, ‘l’ is also known as orbital angular momentum or subsidiary quantum number. Each shell consists of one or more subshells or sublevels. The number of sub-shells in a principal shell is equal to the value of n.
Magnetic orbital quantum number. The fourth quantum number is known as the electron spin quantum number (ms). An electron spins around its own axis, much in a similar way as the earth spins around its own axis while revolving around the sun.
(a) What does magnetic orbital number describe?
(b) Write the value of four quantum numbers for the valence electron of the sodium atom.
(c) Out of 6s and 4f orbitals, which has higher energy and why?
(d) What is the total number of orbitals associated with the principal quantum number n = 3 ?
Q. 88 Read the passage given below and answer the following questions:
In 1830, Michael Faraday showed that if electricity is passed through a solution of an electrolyte, chemical reactions occurred at the electrodes, which resulted in the liberation and deposition of matter at the electrodes. In the mid-1850s Faraday began to study electrical discharge in partially evacuated tubes, known as cathode ray discharge tubes. When sufficiently high voltage is applied across the electrodes, current starts flowing through a stream of particles moving in the tube from the negative electrode to the positive electrode. These were called cathode rays or cathode ray particles. J.J. Thomson measured the ratio of electrical charge (e) to the mass of the electron (me ) by using a cathode ray tube and applying electrical and magnetic fields perpendicular to each other as well as to the path of electrons. Positively charged particle was characterised in 1919. Later, a need was felt for the presence of electrically neutral particles as one of the constituents of the atom.
(a) What is the value of charge to mass ratio (e/m) of electrons?
(b) How is it concluded that electrons are a universal constituent of all matter?
(c) Which fundamental property of an atom is not understood if we assume that an atom consists of a nucleus containing protons only and an extra nuclear part containing an equal number of electrons?
(d) Calculate the total no. of electrons present in one mole of methane.
Q. 89 What were the drawbacks of Rutherford’s model of atom? Describe Bohr’s model of atom and explain its usefulness over the Rutherford’s model.
Q. 90 In a hydrogen spectrum if electron moves from 6th to 3rd orbit by transition in multi steps then find out the following steps
(a) Total number of lines in spectrum
(b) Total number of lines in UV region
(c) Total number of lines in visible region
(d) Total number of lines in IR region
Q. 91 When an electric discharge is passed through hydrogen gas, the hydrogen molecules dissociate to produce excited hydrogen atoms.
These excited atoms emit electromagnetic radiation of discrete frequencies which can be given by the general formula.
v– = 109677[1/n12 – 1/n22]
What points of Bohr’s model of an atom can be used to arrive at this formula? Based on these points derive the above formula giving description of each step and each term.
Q. 92 (a) i. An atomic orbital has n = 3. What are the possible values of I and mI?
ii. List the quantum numbers (mI and I) of electrons for 3d orbital.
iii. Which of the following orbitals are possible? 1p, 2s, 2p and 3f
(b). An atom of an element contains 29 electrons and 35 neutrons. Deduce i. The number of protons and
ii. The electronic configurations of the element
Q. 93 What were the limitations of Bohr’s model of atoms? Briefly describe the quantum mechanical model of atom which removes the anomalies present in Bohr model .
Q. 94 (a) State and explain the following: i. Aufbau principle ii. Pauli exclusion principle iii. Hund’s rule of maximum multiplicity.
(b) A gas absorbs a photon of 355 nm and emits at two wavelengths. If one of the emissions is at 680 nm, at what place the other is?
